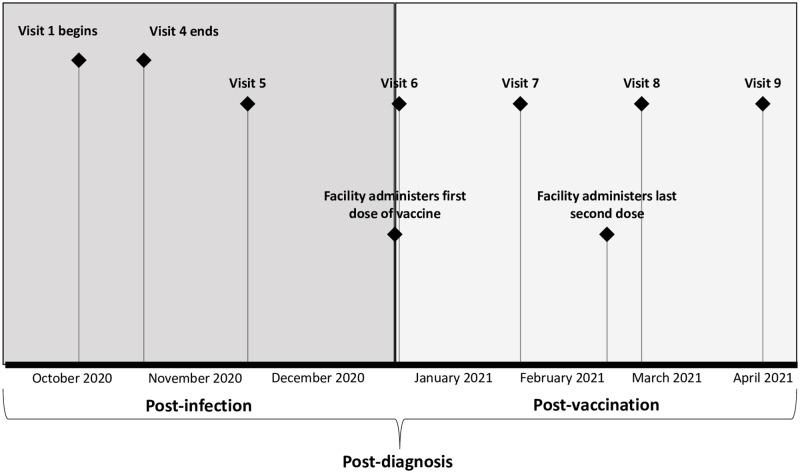
Fig 1. Timeline of participant visits and evaluation activities conducted, by time period—Georgia, October 2020‒April 2021.
Upon enrollment after a SARS-CoV-2–positive diagnosis by BinaxNOW™ COVID-19 Ag Cards and/or real-time reverse transcription polymerase chain reaction, the first four visits were conducted every other day. Visits 5–9 occurred monthly. The post-diagnosis period was defined as the time after each participant’s first positive SARS-CoV-2 test result. The post-infection period was defined as the time from SARS-CoV-2 diagnosis to receipt of first Pfizer-BioNTech COVID-19 Vaccine dose. The post-vaccination period was defined as the time after receipt of first vaccine dose to the end of the evaluation period. Nine participants received their two vaccine doses 21 days apart; one participant received their two vaccine doses 28 days apart.