Abstract
Background
There is a necessity for an optimal COVID-19 vaccination strategy for vulnerable population groups, including people with autoimmune inflammatory arthritis on immunosuppressants such as methotrexate, which inhibit vaccine-induced immunity against SARS-CoV-2. Thus, we aimed to assess the effects of withholding methotrexate for 2 weeks after each dose of ChAdOx1 nCov-19 (Oxford–AstraZeneca) vaccine (MIVAC I) or only after the second dose of vaccine (MIVAC II) compared with continuation of methotrexate, in terms of post-vaccination antibody titres and disease flare rates.
Methods
MIVAC I and II were two parallel, independent, assessor-masked, randomised trials. The trials were done at a single centre (Dr Shenoy's Centre for Arthritis and Rheumatism Excellence; Kochi, India) in people with either rheumatoid arthritis or psoriatic arthritis with stable disease activity, who had been on a fixed dose of methotrexate for the preceding 6 weeks. Those with previous COVID-19 or who were positive for anti-SARS-CoV-2 nucleocapsid antibodies were excluded from the trials. People on high-dose corticosteroids and rituximab were also excluded, whereas other disease-modifying antirheumatic drugs were allowed. In MIVAC I, participants were randomly assigned (1:1) to stop methotrexate treatment for 2 weeks after each vaccine dose or to continue methotrexate treatment. In MIVAC II, participants who had continued methotrexate during the first dose of vaccine were randomly assigned (1:1) to withhold methotrexate for 2 weeks after the second dose of vaccine or to continue to take methotrexate. The treating physician was masked to the group assignments. The primary outcome for both MIVAC I and MIVAC II was the titre (absolute value) of anti-receptor binding domain (RBD) antibody measured 4 weeks after the second dose of vaccine. All analyses were done per protocol. The trials were registered with the Clinical Trials Registry- India, number CTRI/2021/07/034639 (MIVAC I) and CTRI/2021/07/035307 (MIVAC II).
Findings
Between July 6 and Dec 15, 2021, participants were recruited to the trials. In MIVAC I, 250 participants were randomly assigned and 158 completed the study as per the protocol (80 in the methotrexate hold group and 78 in the control group; 148 [94%] were women and 10 [6%] were men). The median post-vaccination antibody titres in the methotrexate hold group were significantly higher compared with the control group (2484·0 IU/mL, IQR 1050·0–4388·8 vs 1147·5 IU/mL, 433·5–2360·3; p=0·0014). In MIVAC II, 178 participants were randomly assigned and 157 completed the study per protocol (76 in the methotrexate hold group and 81 in the control group; 135 [86%] were women and 22 [14%] were men). The methotrexate hold group had higher post-vaccination antibody titres compared with the control group (2553·5 IU/ml, IQR 1792·5–4823·8 vs 990·5, 356·1–2252·5; p<0·0001). There were no reports of any serious adverse events during the trial period.
Interpretation
Withholding methotrexate after both ChAdOx1 nCov-19 vaccine doses and after only the second dose led to higher anti-RBD antibody titres compared with continuation of methotrexate. However, withholding methotrexate only after the second vaccine dose resulted in a similar humoral response to holding methotrexate after both vaccine doses, without an increased risk of arthritis flares. Hence, interruption of methotrexate during the second dose of ChAdOx1 nCov-19 vaccine appears to be a safe and effective strategy to improve the antibody response in patients with rheumatoid or psoriatic arthritis.
Funding
Indian Rheumatology Association.
Research in context.
Evidence before this study
Methotrexate is known to adversely affect vaccine-induced immunity. We searched PubMed and Scopus for randomised controlled trials published between database inception and July 3, 2022, using the terms ([methotrexate] AND [vaccine] AND [influenza OR covid-19 OR SARS-CoV-2]) AND (Rheumatic diseases]), with no language restrictions. Trials that investigated the effect of holding methotrexate after vaccination on vaccine responses were identified. Our search identified two randomised trials from South Korea, one that investigated a 2-week methotrexate hold versus continuation after the vaccine dose, and another that evaluated a 4-week hold of methotrexate before and after vaccination and a 2-week hold each before and after vaccination and continuation of methotrexate after vaccination. A temporary methotrexate hold improved the humoral immune responses to influenza vaccination in both trials. Increasing the methotrexate hold to 4-weeks did not improve the outcomes. A randomised trial from Brazil reported an improved anti-receptor binding domain antibody response against the SARS-CoV-2 spike protein with a 2-week methotrexate hold after each dose of the CoronaVac vaccine (Sinovac Biotech; Beijing, China). However, the study was prone to bias due to a greater dropout rate in the methotrexate hold group versus the methotrexate continuation group, as disease flare was considered a criterion for exclusion. Another randomised trial from the UK analysed the effect of a 2-week methotrexate hold versus continuation after the third dose of vaccine (ChAdOx1 nCoV-19 and mRNA vaccines) and found a significantly higher antibody response after the 2-week methotrexate hold. However, physician-assessed disease flares using standard outcome measures were not assessed. Finally, a randomised trial from Australia assessed the immunogenicity of COVID-19 vaccines by withholding conventional, biologic, or targeted synthetic disease-modifying antirheumatic drugs (DMARDs) for 1–2 weeks after the first dose of vaccine only and found that withholding targeted synthetic DMARDs yielded better antibody responses compared with a control group. However, the current sample size did not have adequate power for subgroup analysis to look at individual effects of different DMARDs that had been co-prescribed with methotrexate.
Added value of this study
Before the trials presented here, the evidence supporting withdrawal of methotrexate at the time of COVID-19 vaccination was scarce, especially for the ChAdOx1 nCoV-19 vaccine. In MIVAC I, in which methotrexate was withheld after each of two vaccine doses, the post-vaccination antibody titre was higher in individuals in which methotrexate was withdrawn compared with those who continued methotrexate, but methotrexate withdrawal led to an increased number of disease flares. In MIVAC II, in which methotrexate was withheld only after the second vaccine dose, the antibody titre was higher in individuals in whom methotrexate was withdrawn compared with the control group, without an increased risk of disease flares.
Implications of all the available evidence
Withholding methotrexate after COVID-19 vaccination led to a higher antibody response. However, withholding methotrexate only after the second vaccine dose appeared to carry a lower risk for disease flare, with no considerable difference in the humoral response compared with when methotrexate is withheld for both vaccine doses. This finding remained despite the wide gap between the two doses of the ChAdOX1 n COV 19 vaccine (12–16 weeks) and could be translated into practice by withholding methotrexate only during the second dose of vaccine to achieve an optimal trade-off between immunogenicity and risk of flare. Future studies should measure how this change in practice affects the risk of breakthrough infections during booster doses in a real-life scenario.
Introduction
Deployment of COVID-19 vaccines has become an effective countermeasure to the poor health outcomes associated with SARS-CoV-2 infection, especially in people with immune-mediated inflammatory diseases who might be at increased risk of severe COVID-19 outcomes compared with the general population. Existing evidence supports vaccination against SARS-CoV-2 in people with immune-mediated inflammatory diseases, as the benefits of vaccination outweigh the risk for adverse events and disease flares.1, 2, 3 The ChAdOx1 nCoV-19 (Oxford–AstraZeneca) COVID-19 vaccine, manufactured by the Serum Institute of India, is the predominant vaccine that has been used in India. The recommended dosing schedule is two doses administered at an interval of 12–16 weeks.
People on long-term immunosuppressants were excluded from COVID-19 vaccine trials, including those for the ChAdOx1 nCoV-19 vaccine.4 Methotrexate is the anchor drug for various immune-mediated inflammatory diseases. However, methotrexate can reduce humoral immune responses via interactions with B-cell activation factor, generation of immunoregulatory adenosine, and induction of regulatory B cells.5 Temporary interruption of methotrexate for 2 weeks and 4 weeks during influenza vaccination significantly improved vaccine immunogenicity in people with rheumatoid arthritis but increased the risk of a disease flare.6 The results from the influenza trial formed the basis of evidence for the American College of Rheumatology (ACR) recommendations to hold methotrexate treatment during COVID-19 vaccination.7 As newer evidence comes to light, the ACR and other rheumatology societies have been updating their clinical guidance,8, 9 and recommend that most oral immunomodulatory drugs, including methotrexate, should be withheld for 1–2 weeks after each dose of COVID-19 vaccine if disease activity allows.
Evidence from observational studies shows an impaired humoral response to BNT162b2 mRNA COVID-19 vaccine in people on methotrexate.10, 11, 12, 13 However, a longitudinal cohort study showed that the humoral response to COVID-19 vaccines is preserved in people on methotrexate, but with lower levels of anti-spike antibody.14 Thus, the available quality of evidence to support the recommendation of withholding methotrexate is low. Hence, we designed two randomised controlled trials to compare the effects and safety of withholding methotrexate on the humoral immune response to vaccination. The first trial (MIVAC I) was to study the effects of withholding methotrexate after both doses of the ChAdOx1 nCov19 (Oxford–AstraZeneca) vaccine. The second trial (MIVAC II) explored the effects of withholding methotrexate only after the second dose of the ChAdOx1 nCov-19 vaccine.
Methods
Study design and participants
The MIVAC trials were two independent, parallel, assessor-masked, randomised controlled trials that were done at a single centre (Dr Shenoy's Centre for Arthritis and Rheumatism Excellence; Kochi, India). People who attended CARE between July 6 and Dec 15, 2021, were recruited to the trials. People with a diagnosis of rheumatoid arthritis according to ACR criteria15 or psoriatic arthritis according to Classification Criteria for Psoriatic Arthritis criteria,16 aged 18 years or older, and who were on a stable dose of methotrexate for at least 6 weeks were eligible for enrolment in the two trials. People who were on corticosteroids of more than 5 mg prednisolone or equivalent doses, or who had received rituximab in the past year, were excluded from the trials.
All potential participants were screened, and those who had known COVID-19 in the past or presence of anti-SARS-CoV-2 nucleocapsid antibodies were excluded. For MIVAC I, all participants with positive anti-receptor binding domain (RBD) titres at baseline were also excluded. Other exclusion criteria for both of the trials were acute febrile illness or symptoms of COVID-19 before vaccination, being a primary contact of SARS-CoV-2 RT-PCR-positive cases, previous history of allergy to vaccine components, previous acute Guillain-Barré syndrome or demyelinating syndrome, live vaccine receipt within the past 4 weeks, inactivated vaccine receipt in the past 2 weeks, and plans to become pregnant in the next few months.
The trials were conducted according to Good Clinical Practice guidelines and reported according to CONSORT recommendations. The trials were approved by the ethics committee of Sree Sudheendra Medical Mission (Kochi, India; reference numbers MIVAC I IEC/2021/39 and MIVAC II IEC/2021/40). Participants gave written informed consent before participating in the trials. The study protocol is available in the appendix (pp 10–21).
Randomisation and masking
In MIVAC I, participants were randomly assigned (1:1) to stop methotrexate treatment for 2 weeks after each vaccine dose or to continue methotrexate treatment. In MIVAC II, participants who had continued methotrexate during the first vaccine dose were randomly assigned (1:1) to stop methotrexate treatment for 2 weeks after the second vaccine dose or to continue methotrexate treatment. Participants who did not follow the randomisation pattern, such as those who held methotrexate for 1 week rather than 2 weeks or who held methotrexate instead of continuing the drug, were considered to represent protocol deviations and were excluded from the per protocol analysis.
Enrolment of participants was done by ASr and RU. Randomisation was done by TGS and SnJ using a permuted block randomisation method with a block size of four. The allocation sequence was generated by an online tool called the sealed envelope, prepared by an independent statistician. Investigators who assessed disease activity (PS), treating physicians, statisticians, and laboratory staff were masked to participant allocation. To measure adherence to the intervention, participants were required to note methotrexate administration in a diary.
Procedures
In MIVAC I, unvaccinated participants in the methotrexate hold group were instructed to discontinue methotrexate treatment for 2 weeks and resume their previous dose on the third week following the first and second dose of the ChAdOx1 nCov-19 vaccine. Participants in the control group were instructed not to omit methotrexate treatment after the first and second doses of the vaccine. Two unmasked researchers (TGS and SnJ) followed up by telephone call 2 weeks after each vaccine dose to record protocol adherence and adverse events. Anti-RBD titres were measured at baseline (visit 0) and subsequently at 4 weeks after each dose of vaccine (visits 1 and 2). Disease flare was assessed 4 weeks after each dose of the vaccine by a masked researcher.
In MIVAC II, participants in the methotrexate hold group were instructed to discontinue methotrexate for 2 weeks after the second dose of the vaccine. Participants in the control group were instructed to continue taking the same dose of methotrexate after the second dose of the vaccine. Two unmasked researchers (TGS and SnJ) followed up by telephone call 2 weeks after the second vaccine dose to ensure protocol adherence and adverse events. Anti-RBD titres and disease flare were assessed 4 weeks after the second dose of vaccine (visit 2). Flare was assessed by Disease activity score-28 (DAS28), Clinical Disease Activity Index for Psoriatic Arthritis, or physician's intent to increase the dose of disease-modifying anti-rheumatic drugs (DMARDs). Rescue treatment using a short course of corticosteroids or non-steroidal anti-inflammatory drugs (NSAIDs) was permitted in both trials at the treating physician's discretion.
All participants were given a mobile telephone number to contact if they developed any symptoms suggestive of COVID-19. During telephone follow-up, participants were asked about symptoms suggestive of COVID-19. If they reported any symptoms of COVID-19 or being a primary contact of someone with PCR-positive SARS-CoV-2 infection, they were encouraged to test for SARS-CoV-2 by RT-PCR or antigen test from a regional Indian Council of Medical Research-approved laboratory. If a participant tested positive for SARS-CoV-2 by these methods, they would be excluded from further analysis. Participants who developed COVID-19 were excluded from the study as this could confound the primary outcome and might predispose them to additional disease flares.
Serum samples were drawn at 4 weeks after each dose of vaccine. IgG antibodies against the RBD of the spike protein were measured by ELISA using the Elecsys kit (Roche; Basel, Switzerland) as per the manufacturer's instructions. Anti-nucleocapsid antibodies were measured using Elecsys anti-SARS-CoV-2 electrochemiluminescence immunoassay (Roche) as per the manufacturer's instructions.
Outcomes
The primary outcome for both MIVAC I and MIVAC II was the titre (absolute value) of anti-RBD antibody measured 4 weeks after the second dose of vaccine. The secondary outcomes were the proportions of participants who had a disease flare after randomisation, and the proportion of participants with anti-RBD antibodies 4 weeks after the second dose (ie, seroconversion). Although SARS-CoV-2 neutralisation assay was a predefined secondary outcome, we did not ultimately assess this, as subsequent data from our centre revealed that anti-RBD antibodies were a good predictor of breakthrough SARS-CoV-2 infections with good correlation with neutralisation.12 Disease flare was predefined as an increase in disease activity scores or a physician's intent to increase the dose of DMARDs within 4 weeks of vaccination. For participants with rheumatoid arthritis, the predefined increase in disease activity was an increase in DAS28 greater than 1·2 (irrespective of the baseline DAS28) or greater than 0·6 if the baseline DAS28 was at least 3·2. For participants with psoriatic arthritis, the predefined increase in disease activity was defined as Clinical Disease Activity Index for Psoriatic Arthritis greater than 13.17, 18, 19
Adverse events that occurred at any time during the trial period were captured from the participants at each visit, as well as during telephone follow-up. The severity of adverse events were classified according to WHO definition.20
Statistical analysis
In our previous study (unpublished), the mean antibody titre of participants who were on methotrexate (with or without hydroxychloroquine) who had received two doses of ChAdOx1 nCov-19 vaccine was 948 IU (SD 563). Considering the effect of withholding methotrexate to increase antibody titres by at least 30% with an α of 0.05 and power of 0·8, the number of participants required in our trial was 124 (62 per group).
For MIVAC I, where study inclusion took place just before the first dose of ChAdOx1 nCov-19 vaccine and the follow-up duration was 4 months (3 months up to the second vaccine dose and 1 month up to final antibody measurement), we presumed an attrition rate of 50% (40% catching COVID-19 and 10% dropout), resulting in a calculated sample size of 248 participants.
For MIVAC II, where study inclusion took place before the second dose of vaccine and follow-up was 1 month, we presumed an attrition rate of 30% (20% catching COVID-19 and 10% dropout), resulting in a calculated sample size of 178 participants.
The normality of data was assessed by the Shapiro-Wilk test. For the primary outcome, antibody titres were compared between the trial group and the control group with Mann-Whitney U tests. For arthritis flares and adverse effects, the Fisher's exact test was used to compare proportions between the study groups. All analyses were done per protocol.
A post-hoc multivariable analysis was done to examine which factors affected final antibody titres. We constructed an ANCOVA model with final antibody titres as the dependent variable, age, and methotrexate dose as covariates, and group (methotrexate hold vs control), disease (rheumatoid arthritis or psoriatic arthritis), and use of hydroxychloroquine, sulfasalazine, tofacitinib, or low-dose corticosteroid as fixed factors.
All analysis and data visualisation was done in R version 4.2. The trials were registered with the Clinical Trials Registry- India, number CTRI/2021/07/034639 (MIVAC I) and CTRI/2021/07/035307 (MIVAC II).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
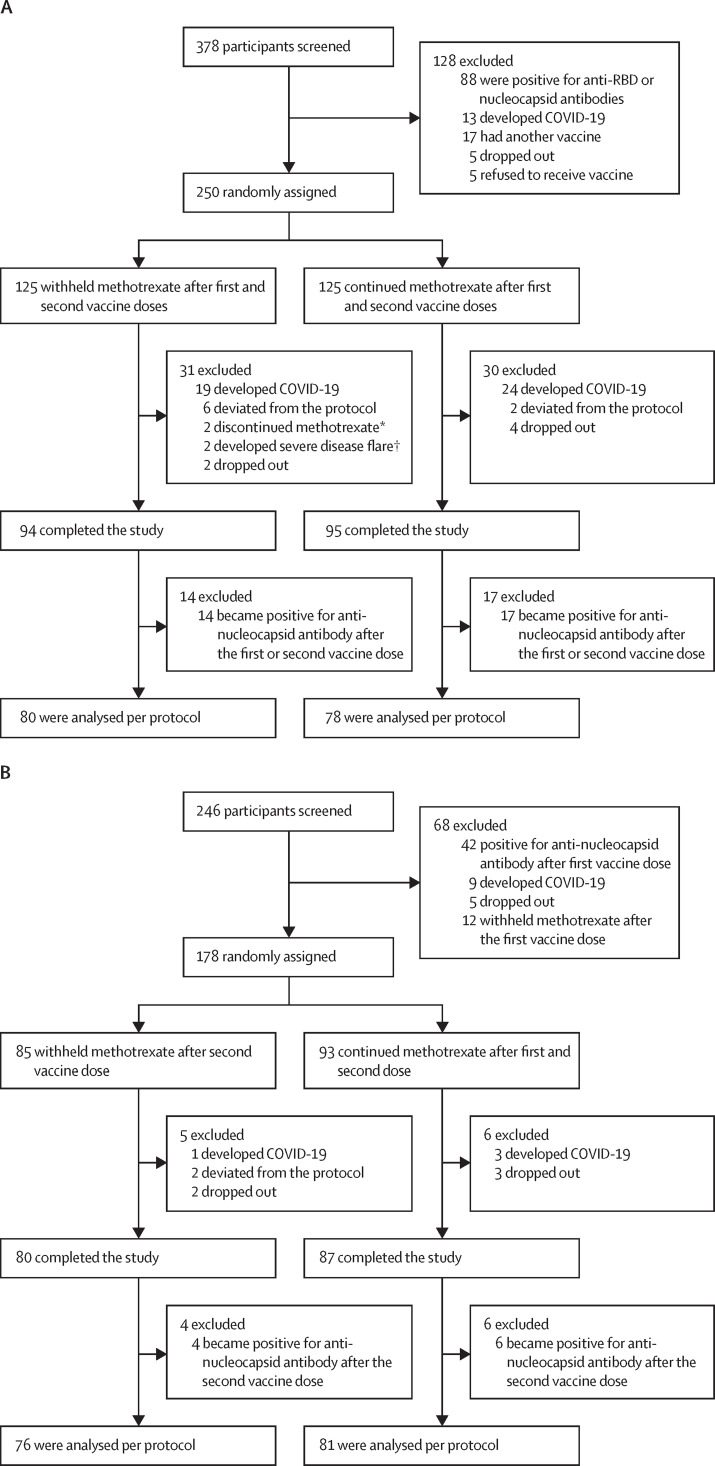
In MIVAC I, between July 6 and Dec 7, 2021, 250 participants were recruited and randomly assigned to the two study groups. 158 participants were included in the per protocol analysis (80 in the methotrexate hold group and 78 in the control group; figure 1A ). In MIVAC II, between July 30 and Dec 15, 2021, 178 participants were recruited and randomly assigned to the two study groups. 157 participants were included in the per protocol analysis (76 in the methotrexate hold group and 81 in the control group; figure 1B).
Figure 1.
Trial profiles for MIVAC I (A) and MIVAC II (B)
*Two patients discontinued methotrexate because of adverse events (elevated transaminases). †Severe disease flare was defined as a flare that was not resolved before the second dose of the vaccine, despite increased disease-modifying antirheumatic drug dose and rescue therapy.
The study groups were similar in terms of baseline demographics, disease activity, and treatment, including methotrexate and corticosteroid doses, in the per-protocol analysis (table 1 ) and at randomly assignment (appendix p 1) in MIVAC I and MIVAC II. In MIVAC I, sulfasalazine (25 [16%] of 158 participants), leflunomide (12 [8%] participants), tofacitinib (six [4%] participants), and anti-tumour necrosis factor (TNF; two [1%] participants] were co-prescribed with methotrexate. In MIVAC II, sulfasalazine (24 [15%] of 157 participants), leflunomide (18 [11%] participants), tofacitinib (five [3%] participants), and anti-TNF (one [1%] participant) were co-prescribed with methotrexate. The median interval between first and second vaccine doses was 91 days (IQR 86–101) in MIVAC I and 92 days (87–98) in MIVAC II (p=0·99)
Table 1.
Baseline demographics of per protocol population
|
MIVAC I |
MIVAC II |
||||
|---|---|---|---|---|---|
| Hold methotrexate (n=80) | Continue methotrexate (n=78) | Hold methotrexate (n=76) | Continue methotrexate (n=81) | ||
| Age, years | 48 (38–53) | 49 (39–59) | 53 (42–59) | 53 (50–62) | |
| Sex | |||||
| Female | 73 (91%) | 75 (96%) | 65 (86%) | 70 (86%) | |
| Male | 7 (9%) | 3 (4%) | 11 (14%) | 11 (14%) | |
| Diagnosis | |||||
| Rheumatoid arthritis | 69 (86%) | 72 (92%) | 70 (92%) | 80 (99%) | |
| Psoriatic arthritis | 11 (14%) | 6 (8%) | 6 (8%) | 1 (1%) | |
| Comorbidities | 27 (34%) | 25 (32%) | 28 (37%) | 33 (41%) | |
| Interval between first and second vaccine doses, days | 90 (85–88) | 93 (87–104) | 91 (88–95) | 92 (86–103) | |
| DAS28-erythrocyte sedimentation rate | 2·7 (2·4–3·2) | 2·6 (2·0–3·3) | 2·7 (2·3–3·4) | 2·8 (2·1–3·5) | |
| cDAPSA | 2·0 (3·0–4·5) | 2·5 (1·3–3·8) | 3·0 (2·8–3·0) | 3·0* | |
| MI (kg/m2) | 23·2 (20·9–25·7) | 22·6 (19·7–26·1) | 24·1 (21·8–26·7) | 24·2 (21·5–27·4) | |
| Drug treatment | |||||
| Hydroxychloroquine | 64 (80%) | 65 (83%) | 62 (82%) | 66 (81%) | |
| Sulfasalazine | 10 (13%) | 15 (19%) | 10 (13%) | 14 (17%) | |
| Leflunomide | 8 (10%) | 4 (5%) | 10 (13%) | 8 (10%) | |
| Tofacitinib | 1 (1%) | 5 (6%) | 2 (3%) | 3 (4%) | |
| Anti-tumor necrosis factor | 1 (1%) | 1 (1%) | 0 | 1 (1%) | |
| Methotrexate, mg/week | 17·5 (10·0–25·0) | 15·0 (10·0–20·0) | 15 (9·4–25·0) | 17·5 (7·5–25·0) | |
| Prednisolone† | 29 (36%) | 23 (29%) | 24 (32%) | 26 (32%) | |
| Prednisolone, mg/day | 2·1 (1·2–2·5) | 1·8 (1·1–2·5) | 2·1 (0·8–2·4) | 2·1 (1·4–2·5) | |
Data are median (IQR) or n (%). DAS28=disease activity score in 28 joints· cDAPSA=Clinical Disease Activity Index for Psoriatic Arthritis·
Only one patient with psoriatic arthritis was included in this group·
Prednisolone dose ≤5 mg or equivalent dose of another corticosteroid.
In MIVAC I, participants who withheld methotrexate after both vaccine doses had significantly higher median antibody titres compared with participants who continued methotrexate after both vaccine doses (2484·0 IU/mL, IQR 1050·0–4388·8 vs 1147·5 IU/mL, 433·5–2360·3; p=0·0014; table 2 ). Three (4%) of 78 participants remained seronegative in the methotrexate continuation group versus no participants in the methotrexate hold group (p=0·12; table 2). In a post-hoc multivariable analysis, to examine which factors affected final antibody titres, only the group (methotrexate hold vs control; p=0·0041) and disease (rheumatoid arthritis vs psoriatic arthritis; p=0·012) were independently associated with final antibody titre.
Table 2.
Immunogenicity after methotrexate hold or continuation and vaccination with ChAdOx1 nCoV-19
|
MIVAC I |
MIVAC II |
|||||
|---|---|---|---|---|---|---|
| Hold methotrexate (n=80) | Continue methotrexate (n=78) | p value | Hold methotrexate (n=76) | Continue methotrexate (n=81) | p value | |
| Antibody titres after first vaccine dose, IU/mL | 11·6 (0·4–50·8) | 15·5 (0·4–54·3) | 0·39 | 25·9 (6·6–61·5) | 16·3 (0·4–64·3) | 0·48 |
| Antibody titres after second vaccine dose, IU/mL | 2484·0 (1050·0–4388·8) | 1147·5 (433·5–2360·3) | 0·0014 | 2553·5 (1792·5–4823·8) | 990·5 (356·1–2252·5) | <0·0001 |
| Seroconversion after the first vaccine dose | 50 (63%) | 54 (69%) | 0·78 | 63 (83%) | 63 (78%) | 0·55 |
| Seroconversion after the second vaccine dose | 80 (100%) | 75 (96%) | 0·12 | 76 (100%) | 77 (95%) | 0·12 |
Data are median (IQR) or n (%), unless otherwise indicated.
In MIVAC II, the median antibody titres after both doses of vaccine were significantly higher in the methotrexate hold group compared with the methotrexate continuation group (2553·5 IU /ml, IQR 1792·5–4823·8 vs 990·5 IU/mL, 356·1–2252·5; p<0·0001; table 2). Four (5%) of 81 participants remained seronegative in the methotrexate continuation group versus no participants in the methotrexate hold group (p=0·12). In the post-hoc multivariable analysis, only group (methotrexate hold vs control; p<0·0001) was independently associated with final antibody titre.
In MIVAC I, 20 (25%) of 80 participants in the methotrexate hold group and six (8%) of 78 participants in the methotrexate continuation group had a disease flare after the first vaccine dose (p=0·0050; table 3 ). After the second vaccine dose, 19 (24%) of 80 participants in the methotrexate hold group and ten (13%) of 78 participants in the methotrexate continuation group had a disease flare (p=0·10; table 3). In MIVAC II, we found no significant difference in the proportion of participants with a disease flare between the two study groups (nine [12%] of 76 participants in the methotrexate hold group vs four (5%) of 81 participants in the methotrexate continuation group; p=0·15). Severity of disease flare was measured based on the cutoff values of DAS28-erythrocyte sedimentation rate and Clinical Disease Activity Index for Psoriatic Arthritis (table 3).
Table 3.
Disease flare across the study groups
|
MIVAC I |
MIVAC II |
||||||
|---|---|---|---|---|---|---|---|
| Hold methotrexate (n=80) | Continue methotrexate (n=78) | p value | Hold methotrexate (n=76) | Continue methotrexate (n=81) | p value | ||
| Flare after first vaccine dose | 20 (25%) | 6 (8%) | 0·0050 | .. | .. | .. | |
| Mild | 7 (9%) | 0 | .. | .. | .. | .. | |
| Moderate | 13 (16%) | 6 (8%) | .. | .. | .. | .. | |
| Flare after second vaccine dose | 19 (24%) | 10 (13%) | 0·10 | 9 (12%) | 4 (5%) | 0·15 | |
| Mild | 6 (8%) | 3 (4%) | .. | 4 (5%) | 1 (1%) | .. | |
| Moderate | 13 (16%) | 7 (9%) | .. | 4 (5%) | 3 (4%) | .. | |
| Severe | 0 | 0 | .. | 1 (1%) | 0 | .. | |
Data are n (%), unless otherwise indicated.
Rescue treatment with NSAIDs and corticosteroids was greater in the methotrexate hold group compared with the methotrexate continuation group in MIVAC I and MIVAC II, mirroring disease flares (appendix p 4).
We compared the baseline characteristics of participants who had disease flares and who did not have disease flares after either vaccine dose in MIVAC I and MIVAC II. Only those participants on oral corticosteroids had an increased risk of disease flare in MIVAC I. In MIVAC II, participants with psoriatic arthritis had an increased likelihood of disease flares. Patients with psoriatic arthritis did not show any worsening of psoriasis in either MIVAC I or MIVAC II (appendix pp 5–7).
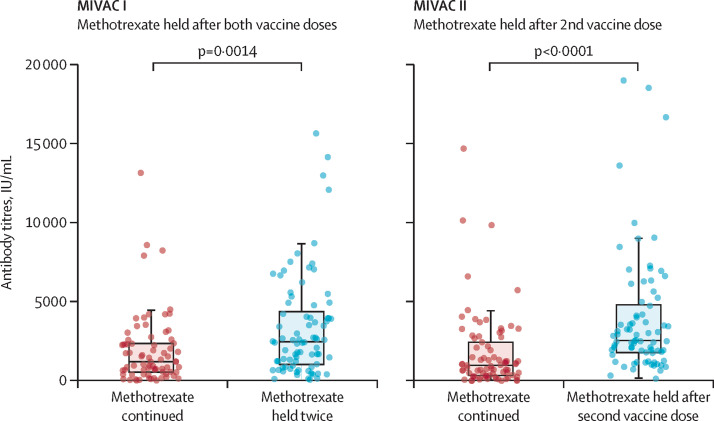
As MIVAC I and II were parallel studies in similar populations, we did a post-hoc comparison of the methotrexate hold groups of MIVAC I (methotrexate held twice) and MIVAC II (methotrexate held only after the second vaccine dose). We found no significant difference in antibody titres between participants who withheld methotrexate twice (MIVAC I) and participants who withheld methotrexate once (MIVAC II; 2484·0 IU/ml, IQR 1050·0–4388·8 in MIVAC I vs 2553·5 IU/ml, 1792·5–4823·8 in MIVAC II; p=0·22). The median antibody titre was higher in the methotrexate hold groups than in the continuation groups (figure 2 ).
Figure 2.
Median antibody titres for methotrexate hold and methotrexate continuation groups in MIVAC I and II
Error bars represent IQRs.
There were no differences in adverse events between the methotrexate hold and control group in MIVAC I and MIVAC II. There were no serious adverse events observed during the trial period (table 4 ).
Table 4.
Adverse events after vaccination (either first or second dose) in MIVAC I and II
|
MIVAC I |
MIVAC II |
|||||
|---|---|---|---|---|---|---|
| Hold methotrexate (n=80) | Continue methotrexate (n=78) | p value | Hold methotrexate (n=76) | Continue methotrexate (n=81) | p value | |
| Injection site reactions | 15 (19%) | 16 (21%) | 0·84 | 13 (17%) | 9 (11%) | 0·35 |
| Fever | 10 (13%) | 11 (14%) | 0·81 | 2 (3%) | 2 (2%) | >0·99 |
| Headache | 10 (13%) | 4 (5%) | 0·16 | 3 (4%) | 5 (6%) | 0·72 |
| Myalgia | 13 (16%) | 7 (9%) | 0·23 | 8 (11%) | 5 (6%) | 0·39 |
| Fatigue | 5 (6%) | 3 (4%) | 0·72 | 1 (1%) | 3 (4%) | 0·62 |
| Vomiting | 1 (1%) | 0 | >0·99 | 0 | 0 | .. |
| Abdominal pain | 1 (1%) | 1 (1%) | >0·99 | 0 | 0 | .. |
Data are n (%).
Post-hoc analysis showed that the median anti-RBD titres in MIVAC I did not differ significantly after the first vaccine dose between the methotrexate hold and methotrexate continuation groups (11·6 IU/mL, IQR 0·4–50·8 vs 15·5 IU/mL, 0·4–54·3; p=0·39; table 2). Combining the results of both trials, seroconversion was higher in the methotrexate hold groups compared with the methotrexate continuation groups (156 [100%] of 156 participants vs 152 [95%] of 159 participants; p=0·015).
The circulating SARS-CoV-2 variant of concern during July to December, 2021, was the delta variant. We did a post-hoc analysis of SARS-CoV-2 breakthrough infections in MIVAC I and MIVAC II since there was a possibility that withholding methotrexate might have led to increased susceptibility to SARS-CoV-2 infection. In MIVAC I, the proportion of participants who developed symptomatic COVID-19 after the first and second vaccine dose in the methotrexate hold group versus the methotrexate continuation group was not statistically different (appendix p 8). The proportion of participants who developed asymptomatic COVID-19 after the first and second vaccine dose in the methotrexate hold group versus the methotrexate continuation group was not statistically different. Similar findings were also found in MIVAC II (appendix p 8).
Discussion
We did the MIVAC I trial to assess the effect on immunogenicity and safety of methotrexate discontinuation for 2 weeks after each dose of the ChAdOx1 nCov-19 vaccine. The study showed that anti-RBD antibody titres were higher in the methotrexate hold group compared with the control group, albeit accompanied by an increased risk of disease flare. The MIVAC II trial assessed the effect on immunogenicity and safety of methotrexate discontinuation for 2 weeks after the second dose of the ChAdOx1 nCov-19 vaccine. Anti-RBD titres were again significantly higher in the methotrexate hold group compared with the control group, with no increased risk of disease flare. When the results of both the MIVAC I and MIVAC II trials were compared post-hoc, we found no difference in the anti-RBD titres between methotrexate hold after both vaccine doses versus methotrexate hold after only the second vaccine dose, with the benefit of fewer disease flares in MIVAC II.
Previous studies have suggested that immuno-suppression impairs humoral responses to influenza, pneumococcal, and hepatitis B vaccines.21 Data on the immunogenicity of COVID-19 vaccines also suggest impaired humoral and cellular immunity with methotrexate, mycophenolate mofetil, and B-cell depleting therapy.10, 14 As methotrexate is the most widely used drug in the treatment of immune-mediated inflammatory diseases, it is imperative to identify its effect on immunogenicity.
The MIVAC I trial showed a significant beneficial effect on immunogenicity of a 2-week methotrexate discontinuation after both ChAdOx1 nCov-19 vaccine doses. Similar observations were reported in a randomised trial in people with rheumatoid arthritis on stable doses of methotrexate receiving the CoronaVac (Sinovac Life Sciences) vaccine.22 That trial found a 25% improvement in antibody titres after CoronaVac vaccination with a temporary methotrexate withdrawal.22
One retrospective study in older people with immune-mediated inflammatory diseases receiving multiple COVID-19 vaccines showed that participants with a 7-day methotrexate hold developed higher mean neutralising antibody titres than did those who continued the drug.23 An open-label trial from the UK found significantly higher anti-RBD antibody titres in a 2-week methotrexate hold group versus continuation group in people with immune-mediated inflammatory diseases receiving their third vaccine dose.24 Our study, which had a longer interval between the two vaccine doses than these previous studies, showed a more than 80% antibody titre improvement with 2 weeks of methotrexate hold after the ChAdOx1 nCov-19 vaccination.
By contrast, a randomised trial in Australia did not find a benefit of methotrexate hold after the first dose of ChAdOx1 nCov-19 or BNT162b2 mRNA COVID-19 vaccines, except in a subgroup of patients receiving targeted synthetic DMARDs. This finding could be due to the heterogeneity in the type of vaccine used, the participant cohort, or variability in the duration of methotrexate hold.25
The results of the MIVAC trials are in concordance with a previous influenza vaccine trial,6 which formed the basis for the ACR's recommendation to hold methotrexate with COVID-19 vaccination. The influenza trial used seroconversion as the primary outcome measure. Since data on the ChAdOx1 nCov-19 vaccine and our previous experience showed that seroconversion was observed in almost 99% of vaccine recipients, we did not use this as the primary outcome measure.4, 25, 26, 27 However, if we combine the results of both the trials, seroconversion was statistically increased even if methotrexate was held only after the second dose of vaccine.
Our data revealed that anti-RBD titres and seroconversion rates did not significantly increase, despite holding methotrexate after the first dose of ChAdOx1 nCov-19 vaccine. Previous observational cohort studies have shown that seroconversion rates were lower in people treated with methotrexate after the first COVID-19 vaccine dose.10, 11, 12 This finding contrasts with the findings of the CoronaVac vaccine study,22 which showed a significantly higher seroconversion rate in the methotrexate hold group after the first dose of vaccine. However, MIVAC I was not powered to investigate differences in the humoral response after each vaccine dose, and the final immune response after two vaccine doses is more clinically relevant for protection.
It was important to quantify the risk–benefit ratio of withholding methotrexate for balancing the risk of disease flares versus improving immunogenicity. Discontinuation of methotrexate after COVID-19 vaccination can result in the exacerbation of underlying immune-mediated inflammatory diseases, adding to the risk of disease flare that might be posed by adjuvants, viral proteins, and other vaccine components.28 Evidence from an influenza vaccine trial showed that a 2-week methotrexate hold was non-inferior to a 4-week hold, and thus we used a 2-week hold in our study.6
In MIVAC I, the methotrexate hold group had a higher frequency of disease flares after the first vaccine dose. The number of flares did not significantly increase after the second dose of vaccine in either MIVAC I or II. This finding was again in contrast to the CoronaVac vaccine study,22 which reported more disease flares after the second vaccine dose. This difference could be due to the shorter interval between CoronaVac doses of 28 days, resulting in the interruption of methotrexate twice in quick succession. Additionally, the disease flare rate after inactivated vaccines could be different from those of adenoviral vaccines.29
Vaccination requires the sequential activation of various immune cells, which might be impaired in the presence of immunomodulators.30 Thus, optimal strategies are required that can increase the immunogenicity of vaccines in people with immune-mediated inflammatory diseases without increasing their risk of disease flare. MIVAC I and II have shown that the optimal strategy for methotrexate might be to withhold treatment only after the second dose of the vaccine. A pooled analysis of both trials revealed that anti-RBD titres were similar for those who discontinued methotrexate only for the second vaccine dose and those who discontinued methotrexate after both vaccine doses. Methotrexate hold might have a variable effect on the basis of age, type of immune-mediated inflammatory disease, concurrent immunosuppression, and comorbidities.31 Our trial was not powered for subgroup analysis to investigate these factors.
Most participants in our study tolerated the ChAdOx1 nCov-19 vaccine well, with no severe adverse events reported. However, the methotrexate hold group had a higher frequency of fever, myalgia, and headache. Since the participants were not masked to study allocation, reporting bias cannot be excluded.
The trial had some limitations. First, India was in its second and third wave of COVID-19 during the trial period, leading to many participants being excluded from the trial due to SARS-CoV-2 infection. However, this event had been anticipated and was within the range of estimated attrition before the start of the trial. Furthermore, these participants could represent those with a greater susceptibility to infection, and hence the study population might not be representative of all people with immune-mediated inflammatory diseases on methotrexate. Second, people on corticosteroid doses greater than 5 mg and rituximab were excluded from the trial. Therefore, a subset of people with severe rheumatoid arthritis was not included in this study. Nevertheless, recommendations suggest that DMARDs should not be withheld in people with severe disease. Third, we compared the MIVAC I and II and found no difference between withholding methotrexate once rather than twice. Although the study was not designed for such a comparison, the design of the studies and participants were similar and the studies were done in parallel in the same geographical area. Due to the similar proportion of SARS-CoV-2 infection in both study groups in MIVAC I and II, the probability of potential bias in terms of the final immunogenicity findings is scarce. Fourth, the proportion of people with psoriatic arthritis in our studies was low. However, this proportion reflected the real-world situation, in which the number of people with psoriatic arthritis is lower than the number of people with rheumatoid arthritis. Finally, co-prescription with certain other drugs was allowed, which could affect vaccine immunogenicity.
In conclusion, withholding methotrexate after one or both doses of the ChAdOx1 nCov-19 vaccine in people with autoimmune inflammatory arthritis improved the humoral immunogenicity of the vaccine. Holding methotrexate only after the second dose of vaccine was associated with similar immunogenicity and a lower risk of disease flare compared to withholding methotrexate after both doses of the vaccine.
Data sharing
The data that support the findings of this study are available from the corresponding author on reasonable request to drshenoy@drshenoycare.com. Deidentified participant data will be made available in anonymised form. The study protocol is available in the appendix (pp 10–21). All data will be made available for a period of 3 years after publication. A proposal with a detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability to request our data. The research team will provide an email address for communication once the data are approved to be shared with others. The corresponding author and Dr Shenoy's Centre for Arthritis and Rheumatism Excellence hospital have the right to decide whether to share the data based on these materials. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.
Declaration of interests
SA has received honorarium as a speaker from Pfizer, DrReddy's, Cipla, and Novartis, outside the current work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge Ipca laboratories for providing methotrexate tablets to be used in the study. We also thank the Department of Science Technology for Human Resource at BIRAC (New Delhi, India), and we thank the patients for their participation in the study during the COVID-19 pandemic. The trial is part of a larger project funded by the Indian Rheumatology Association.
Contributors
PS is the guarantor and accepts full responsibility for the work and the conduct of the study. PS affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained. All authors made substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of data for the work; the drafting of the work or revising it critically for important intellectual content; approved the final version for publication; and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. PS is the corresponding author, conceived the study, collected, curated, and analysed the data, devised the study methodology, supervised the study, visualised the data, and wrote the first draft and subsequently reviewed the manuscript. TGS, ASr, and RU collected, curated, and analysed the data and wrote the first draft and subsequently reviewed the manuscript. SA, PM, and AbP collected the data and reviewed the manuscript. SnJ, MM, SEO, JB, AnP, SSB, SN, JG, ASu, AV, SaJ, KKN, and KN reviewed the manuscript. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication. PS, ASr, and RU verified the final data.
Supplementary Material
References
- 1.CDC People with certain medical conditions. Feb 25, 2022. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html
- 2.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis. 2021;80:1330–1338. doi: 10.1136/annrheumdis-2021-220647. [DOI] [PubMed] [Google Scholar]
- 3.Sattui SE, Liew JW, Kennedy K, et al. Early experience of COVID-19 vaccination in adults with systemic rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open. 2021;7 doi: 10.1136/rmdopen-2021-001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bitoun S, Nocturne G, Ly B, et al. Methotrexate and BAFF interaction prevents immunization against TNF inhibitors. Ann Rheum Dis. 2018;77:1463–1470. doi: 10.1136/annrheumdis-2018-213403. [DOI] [PubMed] [Google Scholar]
- 6.Park JK, Lee MA, Lee EY, et al. Effect of methotrexate discontinuation on efficacy of seasonal influenza vaccination in patients with rheumatoid arthritis: a randomised clinical trial. Ann Rheum Dis. 2017;76:1559–1565. doi: 10.1136/annrheumdis-2017-211128. [DOI] [PubMed] [Google Scholar]
- 7.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 1. Arthritis Rheumatol. 2021;73:1093–1107. doi: 10.1002/art.41734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curtis JR, Johnson SR, Anthony DD, et al. American College of Rheumatology guidance for COVID-19 vaccination in patients with rheumatic and musculoskeletal diseases: version 4. Arthritis Rheumatol. 2022;74:e21–e36. doi: 10.1002/art.42109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American College of Rheumatology COVID-19 guidance. https://www.rheumatology.org/Practice-Quality/Clinical-Support/COVID-19-Guidance
- 10.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis. 2021;80:1339–1344. doi: 10.1136/annrheumdis-2021-220597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boekel L, Hooijberg F, van Kempen ZLE, et al. Perspective of patients with autoimmune diseases on COVID-19 vaccination. Lancet Rheumatol. 2021;3:e241–e243. doi: 10.1016/S2665-9913(21)00037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed S, Mehta P, Paul A, et al. Postvaccination antibody titres predict protection against COVID-19 in patients with autoimmune diseases: survival analysis in a prospective cohort. Ann Rheum Dis. 2022;81:868–874. doi: 10.1136/annrheumdis-2021-221922. [DOI] [PubMed] [Google Scholar]
- 13.Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80:1322–1329. doi: 10.1136/annrheumdis-2021-220626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol. 2021;3:e627–e637. doi: 10.1016/S2665-9913(21)00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 16.Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–2673. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 17.Alten R, Pohl C, Choy EH, et al. Developing a construct to evaluate flares in rheumatoid arthritis: a conceptual report of the OMERACT RA Flare Definition Working Group. J Rheumatol. 2011;38:1745–1750. doi: 10.3899/jrheum.110400. [DOI] [PubMed] [Google Scholar]
- 18.van Mens LJJ, van de Sande MGH, van Kuijk AWR, Baeten D, Coates LC. Ideal target for psoriatic arthritis? Comparison of remission and low disease activity states in a real-life cohort. Ann Rheum Dis. 2018;77:251–257. doi: 10.1136/annrheumdis-2017-211998. [DOI] [PubMed] [Google Scholar]
- 19.van der Maas A, Lie E, Christensen R, et al. Construct and criterion validity of several proposed DAS28-based rheumatoid arthritis flare criteria: an OMERACT cohort validation study. Ann Rheum Dis. 2013;72:1800–1805. doi: 10.1136/annrheumdis-2012-202281. [DOI] [PubMed] [Google Scholar]
- 20.WHO Who draft guidelines for adverse event reporting and learning systems. 2005. Available: https://apps.who.int/iris/bitstream/handle/10665/69797/WHO-EIP-SPO-QPS-05.3-eng.pdf
- 21.Arnold J, Winthrop K, Emery P. COVID-19 vaccination and antirheumatic therapy. Rheumatology (Oxford) 2021;60:3496–3502. doi: 10.1093/rheumatology/keab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araujo CSR, Medeiros-Ribeiro AC, Saad CGS, et al. Two-week methotrexate discontinuation in patients with rheumatoid arthritis vaccinated with inactivated SARS-CoV-2 vaccine: a randomised clinical trial. Ann Rheum Dis. 2022;81:889–897. doi: 10.1136/annrheumdis-2021-221916. [DOI] [PubMed] [Google Scholar]
- 23.Arumahandi de Silva AN, Frommert LM, Albach FN, et al. Pausing methotrexate improves immunogenicity of COVID-19 vaccination in elderly patients with rheumatic diseases. Ann Rheum Dis. 2022;81:881–888. doi: 10.1136/annrheumdis-2021-221876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abhishek A, Boyton RJ, Peckham N, et al. Effect of a 2-week interruption in methotrexate treatment versus continued treatment on COVID-19 booster vaccine immunity in adults with inflammatory conditions (VROOM study): a randomised, open label, superiority trial. Lancet Respir Med. 2022;10:840–850. doi: 10.1016/S2213-2600(22)00186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tran AP, Tassone D, Nossent J, Ding NS. Antibody response to the COVID-19 ChAdOx1nCov-19 and BNT162b vaccines after temporary suspension of DMARD therapy in immune-mediated inflammatory disease (RESCUE) RMD Open. 2022;8 doi: 10.1136/rmdopen-2022-002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherian S, Paul A, Ahmed S, et al. Safety of the ChAdOx1 nCoV-19 and the BBV152 vaccines in 724 patients with rheumatic diseases: a post-vaccination cross-sectional survey. Rheumatol Int. 2021;41:1441–1445. doi: 10.1007/s00296-021-04917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shenoy P, Ahmed S, Cherian S, et al. Immunogenicity of the ChAdOx1 nCoV- 19 and the BBV152 vaccines in patients with autoimmune rheumatic diseases. medRxiv. 2021 doi: 10.1101/2021.06.06.21258417. published online June 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bekaryssova D, Yessirkepov M, Zimba O, Gasparyan AY, Ahmed S. Reactive arthritis before and after the onset of the COVID-19 pandemic. Clin Rheumatol. 2022;41:1641–1652. doi: 10.1007/s10067-022-06120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shenoy P, Ahmed S, Paul A, Cherian S, Vijayan A, Babu AS, et al. Inactivated vaccines may not provide adequate protection in immunosuppressed patients with rheumatic diseases. Ann Rheum Dis. 2022;81:295–296. doi: 10.1136/annrheumdis-2021-221496. [DOI] [PubMed] [Google Scholar]
- 30.Grainger R, Kim AHJ, Conway R, Yazdany J, Robinson PC. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol. 2022;18:191–204. doi: 10.1038/s41584-022-00755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feuchtenberger M, Kovacs MS, Eder A, Nigg A, Schäfer A. Methotrexate significantly reduces the humoral vaccination response against SARS-CoV-2 in older but not younger patients with rheumatoid arthritis. Rheumatol Int. 2022;42:959–966. doi: 10.1007/s00296-022-05123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request to drshenoy@drshenoycare.com. Deidentified participant data will be made available in anonymised form. The study protocol is available in the appendix (pp 10–21). All data will be made available for a period of 3 years after publication. A proposal with a detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability to request our data. The research team will provide an email address for communication once the data are approved to be shared with others. The corresponding author and Dr Shenoy's Centre for Arthritis and Rheumatism Excellence hospital have the right to decide whether to share the data based on these materials. After approval of a proposal, data can be shared through a secure online platform after signing a data access agreement.