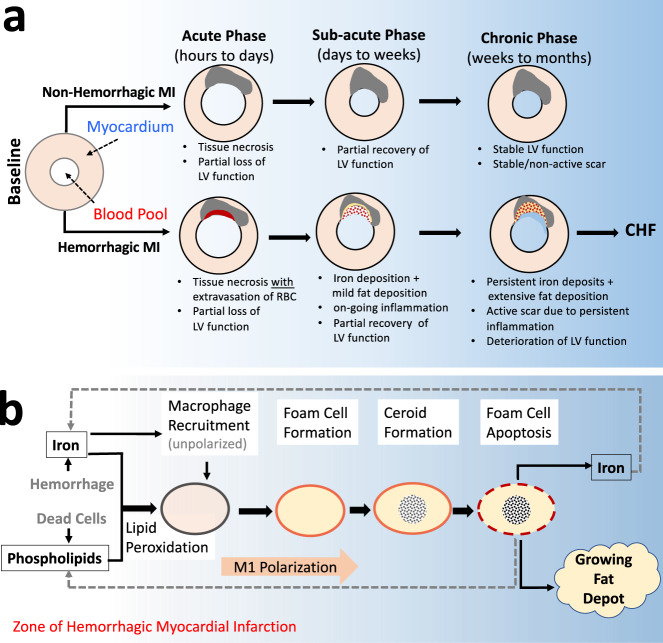
Fig. 1. An overarching model of how hemorrhagic infarction promotes chronic heart failure via fat deposition.
a Following MI, compensatory remodeling promotes partial recovery of LV function in the early weeks following the index event. In hemorrhagic infarction, the extravasated red blood cells promote active inflammation and participate in the formation of fat deposition within the infarction zone. This renders the hemorrhagic MI highly active with respect to functional losses, which define chronic heart failure (CHF). In contrast, non-hemorrhagic MIs are not iron-rich and do not result in prolonged inflammation or promote fat deposition, which stabilizes the infarct zone leading to stable functional remodeling in the chronic phase of infarction. b Iron mediates a recurring cycle of events contributing to fat deposition in the chronic phase of hemorrhagic MI. Iron from hemorrhage promotes the recruitment of unpolarized macrophages and oxidizes the lipids in its vicinity; the oxidized lipid and iron are taken up by the macrophages, which promote their polarization into a pro-inflammatory state and transform into foam cells. The foam cells produce ceroids and destabilize the lysosomes and drive foam cell apoptosis, the remnants of which participate in fat deposition, with the released iron recycled to enter the pathway which perpetuates to continually support inflammation and growth of the fat depot.