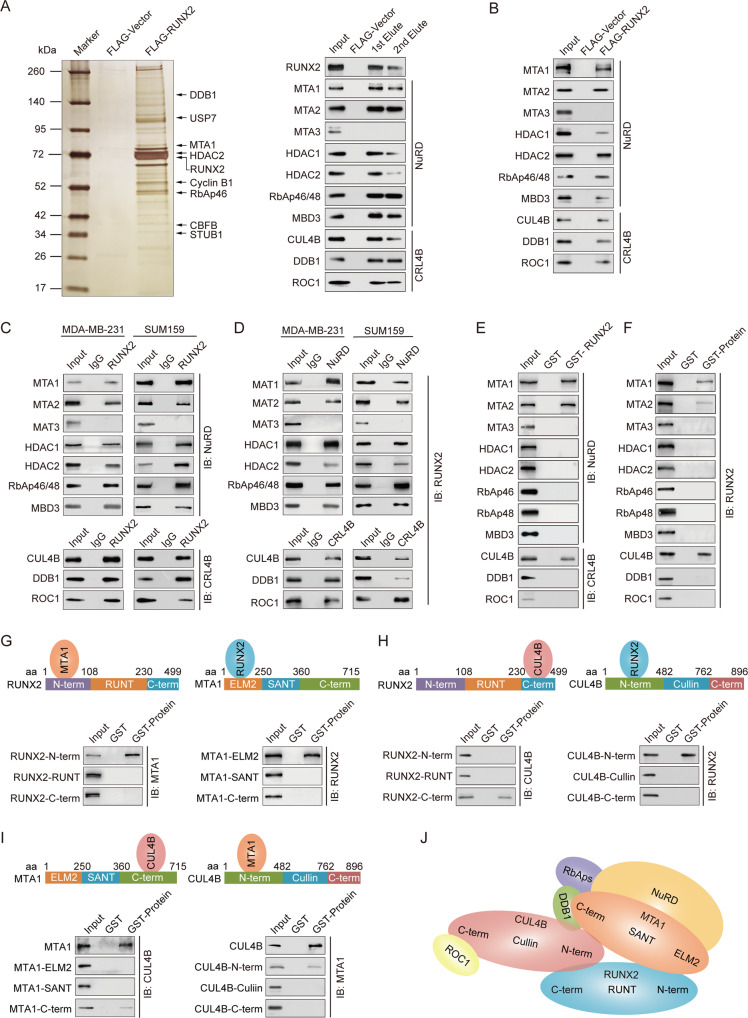
Fig. 2. RUNX2 is physically associated with the NuRD(MTA1) complex and the CRL4B complex.
A Immunoaffinity purification and mass spectrometry analysis of RUNX2-containing protein complexes. Whole-cell extracts from MDA-MB-231 cells stably expressing FLAG-Vector or FLAG-RUNX2 were immunopurified using anti-FLAG affinity columns and eluents with FLAG peptide. Elutes were resolved using SDS-PAGE and silver-stained. Protein bands were retrieved and analyzed using mass spectrometry. B Western blotting analysis of the purified fractions using antibodies against FLAG in MDA-MB-231 cells. C Immunoprecipitation with antibody against RUNX2 followed by immunoblotting with antibodies against MTA1, MTA2, MTA3, HDAC1, HDAC2, RbAp46/48, MBD3, CUL4B, DDB1, and ROC1. D Immunoprecipitation with antibodies against MTA1, MTA2, MTA3, HDAC1, HDAC2, RbAp46/48, MBD3, CUL4B, DDB1, and ROC1 followed by immunoblotting with antibody against RUNX2. E, F GST pull-down assays with GST-fused proteins expressed in bacteria and in vitro translated proteins as indicated. G–I Identification of the essential domains of RUNX2, MTA1, or CUL4B required for interaction. J Schematic diagram depicting molecular interactions among RUNX2/NuRD(MTA1)/CRL4B complex. IB, immunoblotting; aa, amino acid.