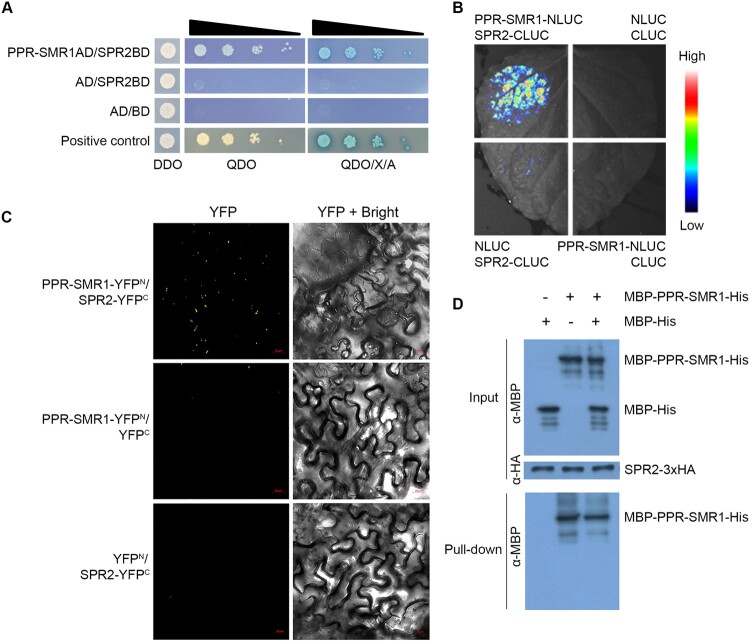
Figure 6.
SPR2 interacts with PPR-SMR1. A, Interaction analysis of SPR2 and PPR-SMR1 in Y2H assays. The combination of PPR-SMR1AD/SPR2BD was co-transfected into yeast strain Y2H Gold and spotted onto SD/–Leu/–Trp (DDO) medium and growth of diploid yeast colonies on SD/–Ade/–His/–Leu/–Trp (QDO) medium and QDO with added the X-α-gal and AbA (QDO/X/A) medium to reveal protein–protein interactions. Positive interaction was verified by growth on QDO and QDO/X/A plates. AD: GAL4 activation domain; BD: GAL4 DNA binding domain. B, LCI assays to determine interactions between SPR2 and PPR-SMR1. The combinations of PPR-SMR1–NLUC/SPR2–CLUC were co-infiltrated into 4-week-old N. benthamiana leaves. The luciferase signals were visualized using the Lumazone FA Pylon2048B system. The intensity of the fluorescent signals represents their interaction activities. C, BiFC analysis for interactions between SPR2 and PPR-SMR1. YFP is split into N-terminus (YFPN) and C-terminus (YFPC). The combination of PPR-SMR1–YFPN/SPR2–YFPC was co-infiltrated into 4-week-old N. benthamiana leaves using Agrobacterium. YFP signals were detected by a confocal laser microscope. Scale bars, 20 μm. D, Semi-in vivo pull-down assays for interactions between SPR2 and PPR-SMR1. Equal amounts of MBP-PPR-SMR1-His and MBP-His were combined with anti-HA magnetic beads preincubated with SPR2-HA. Pulled-down samples were analyzed by immunoblot with anti-MBP and anti-HA antibody. “+” and “−” indicate the presence and absence of corresponding proteins in the reactions, respectively. MBP: maltose binding protein; HA: human influenza hemagglutinin.