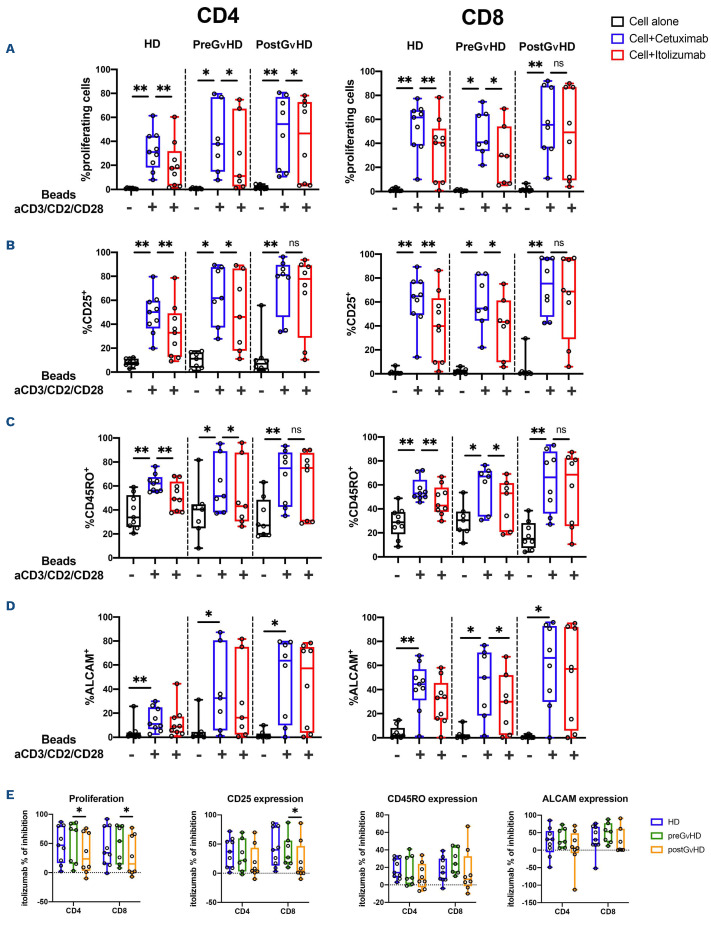
Figure 4.
Inhibition of T-cell proliferation, activation and differentiation by itolizumab. Cryopreserved peripheral blood mononuclear cells (PBMC) obtained from healthy donors (HD) and patients before or after acute graft-versus-host disease (aGvHD) onset were stimulated with anti-CD3/CD2/CD28 beads in the presence of itolizumab or isotype control (cetuximab). (A) Proliferation of CD4 and CD8 T cells measured by CFSE dye dilution; (B) Activation of CD4 and CD8 T cells measured by expression of CD25; (C) maturation of CD4 and CD8 T cells measured by expression of CD45RO; (D) ALCAM expression is absent in resting T cells and is an additional marker of T-cell activation. (E) Percent inhibition induced by itolizumab, comparing activity against HD, pre- and postGvHD samples in CD4 and CD8 T cells. Percentage inhibition was calculated using the following formula: (% cells in isotype control - % cells in itolizumab)/% cells in isotype control. If no difference was observed between isotype control and itolizumab the percentage of itolizumab inhibition equals 0%. Statistically significant differences are noted: **P<0.01; *P<0.05; Wilcoxon rank-sum test. HD n=9, preGvHD n=7, postGvHD n=8.