Abstract
Background
Neutrophil‐to‐lymphocyte ratio (NLR) is an accessible and widely used biomarker. NLR may be used as an early marker of poor prognosis in patients with COVID‐19.
Objective
To evaluate the prognostic value of the NLR in patients diagnosed with COVID‐19.
Methods
We conducted a systematic review and meta‐analysis. Observational studies that reported the association between baseline NLR values (ie, at hospital admission) and severity or all‐cause mortality in COVID‐19 patients were included. The quality of the studies was assessed using the Newcastle‐Ottawa Scale (NOS). Random effects models and inverse variance method were used for meta‐analyses. The effects were expressed as odds ratios (ORs) and their 95% confidence intervals (CIs). Small study effects were assessed with the Egger's test.
Results
We analysed 61 studies (n = 15 522 patients), 58 cohorts, and 3 case‐control studies. An increase of one unit of NLR was associated with higher odds of severity (OR 6.22; 95%CI 4.93 to 7.84; P < .001) and higher odds of all‐cause mortality (OR 12.6; 95%CI 6.88 to 23.06; P < .001). In our sensitivity analysis, we found that 41 studies with low risk of bias and moderate heterogeneity (I 2 = 53% and 58%) maintained strong association between NLR values and both outcomes (severity: OR 5.36; 95% CI 4.45 to 6.45; P < .001; mortality: OR 10.42 95% CI 7.73 to 14.06; P = .005).
Conclusions
Higher values of NLR were associated with severity and all‐cause mortality in hospitalised COVID‐19 patients.
Review criteria
Our systematic review and meta‐analysis included a search strategy from different databases such as EMBASE, SCOPUS, Web of Science, OVID MEDLINE and preprints platforms. Four reviewers independently analysed the titles and abstracts of manuscripts to choose potentially relevant articles. The selected articles were grouped, and duplicates were eliminated with the Rayyan QCRI software. All discrepancies were resolved by group consensus, and finally, the analysis was conducted in RevMan 5.0.
Message for the clinic
The NLR is a biomarker accessible, reproducible and easy to use in COVID‐19 patients. In our study, NLR was strongly associated with a higher odds of severity and all‐cause mortality; NLR could help health professionals to quickly identify high‐risk COVID‐19 patients and adopt low‐cost and timely intervention to prevent complications. This is relevant, especially now, that several countries continue to have a high transmission rate of SARS‐CoV‐2.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is an acute respiratory infection caused by SARS‐CoV‐2. 1 , 2 , 3 On 30 January 2020, the World Health Organization (WHO) declared the epidemic as a public health emergency of international interest. 4 After more than 20 000 cases and 1000 deaths in the European Region, the WHO classified the disease as a pandemic. 5 To date (14 May 2021), more than 162 million cases and 3.37 million deaths have already been reported across the world. 6 According to recent studies, the basic reproduction number (R0) is 3.38, suggesting high transmissibility. 7 Besides the significant human losses, the quarantine and social distancing have had a great impact on the global economy. 8 However, despite the implementation of these strategies, the incidence of cases has been increasing in some countries, and nowadays, some nations are experiencing a second wave.
Sociodemographic and clinical factors, such as older age, male sex, hypertension and diabetes mellitus, increase the mortality rate of COVID‐19. 9 , 10 However, these factors have different distributions between countries. 11 In June 2020, a meta‐analysis reported that the global mortality rate was 2.72% (95% CI 2.19‐4.76). 12 Additionally, a current meta‐analysis reported a 46% (95% CI 18.48‐73.6) prevalence of asymptomatic patients, which has made it difficult to control the pandemic. 12 On the other hand, in symptomatic patients, the most common manifestations are fever, cough, dyspnea, muscle fatigue or muscular pain and chest distress. Moreover, 29.3% of those infected require admission to the intensive care unit (ICU). 12 Regarding the patients admitted to the ICU, reports do not suggest high mortality in them. 13
The neutrophil‐to‐lymphocyte ratio (NLR) is an accessible, reproducible and widely used biomarker for evaluating the prognosis of many health‐related problems such as cardiovascular diseases, various types of cancer, ocular diseases and infectious diseases, among others. 14 , 15 , 16 , 17 , 18 , 19 , 20 The biological basis of this biomarker is related to the response of the innate immune system against systemic inflammation, injury and stress. This is characterised by lymphocytopaenia and neutrophilia. 21 Although there is no consensus on normal cutoff values, two studies reported a cutoff value of 1.65 and 1.70. 22 , 23 Recently, a study showed that NLR is elevated in patients with severe COVID‐19, and the authors suggest that its performance in the prognosis of severe disease should be further evaluated. 24 A brief meta‐analysis, with several limitations, reported that the NLR was a good tool to assess the prognosis of severity in patients with COVID‐19. 25 NLR evaluation can help physicians in initiating treatment and monitoring patient, thereby improving the prognosis and outcomes.
Several studies have evaluated the performance of the NLR in the prognosis of patients with COVID‐19, so it is necessary to synthesise these results to give a more reliable tool for physicians. The objective of this study was to evaluate the prognostic value of the NLR in patients diagnosed with COVID‐19.
2. METHODS
We used the Preferred Reporting Items for Systematic Reviews and Meta‐analysis 26 statement to report our systematic review. A short version of our protocol has been registered in the International Prospective Register of Systematic Review [CRD42020190508].
2.1. Data sources and searches
We searched on 23 December 2020 for studies assessing the association between NLR and clinical outcomes in patients diagnosed with COVID‐19 in the following databases: OVID Medline, OVID Embase, PubMed, Web of Science, Scielo, Scopus, LILACS, Cochrane Library and WHO COVID‐19 Global Research Database. Additionally, a manual search was performed in ScienceDirect, Springer Link, CNKI databases and preprints platforms, such as medRxiv and Scielo Preprints (see Supporting Information Appendix 1). The search strategy was done using the Peer Review of Electronic Search Strategies Checklist. 27 Our team co‐built the search strategy in PubMed, and it was adapted to the other bibliographic databases. We did not apply language restrictions.
2.2. Study selection and data extraction
We included studies that complied the following criteria: (1) prospective or retrospective observational studies (cross‐sectional, case‐control and cohort studies), (2) adult patients (aged > 18 years old) who were diagnosed with COVID‐19, (3) NLR values reported at hospital admission and (4) the association between NLR values and disease severity or other clinical outcomes in COVID‐19 patients was reported. We did not expect to find randomised controlled trials of NLR, as NLR cannot be randomised as interventions. Moreover, we excluded studies that were (1) conducted in animals, (2) duplicated, (3) conference abstracts, (4) case reports, (5) systematic reviews, (6) scoping reviews and (7) editorials or commentaries. Our primary outcome was disease severity, which was defined as meeting at least one of the following criteria: ICU admission, shortness of breath, respiration rate ≥30 times per minute, blood oxygen saturation at rest ≤ 93% and PaO2/FiO2 ≤ 300 mm Hg (ratio of partial pressure of oxygen to fraction of inspired oxygen). However, definitions of severity vary among studies. Mortality was also considered as a secondary outcome.
Four reviewers (IST, JRU, EAB‐B and AAC) independently analysed the titles and abstracts of the selected articles to choose potentially relevant articles. Once the potential literature to be included in our study was found, four authors (IST, JRU, EAB‐B and AAC) independently read the full text of each article selected. If an article did not meet with one or more selection criteria, it was excluded from our study. Discrepancies were resolved by consensus among the team of researchers in each stage. We used Rayyan QCRI software (Qatar Computing Research Institute, Doha, Qatar) to conduct the process of screening and selection of studies. 28 Finally, two authors (IS and JRU) extracted the data from studies through a standardised data extraction sheet made in Microsoft Excel. We extracted the following information: title of the study, first author, year of publication, study design, country and name of the hospital where the study was performed, number of participants, sex, age, comorbidities, stratified sample data, mean or median NLR of the whole sample and according to sample stratification, crude and adjusted association measures, type of outcome and its definition.
2.3. Evaluation of study quality and publication bias
The quality of the studies was assessed with the NOS 29 by two authors. This tool evaluates the quality of published nonrandomised studies and is based on three items: selection, comparability and outcome/exposure. Each item has subitems, on which a star‐based score was assigned. Studies with scores ≥ 6 were considered as having a low risk of bias (high quality), scores of 4‐5 as having a moderate risk of bias, and scores < 4 as having a high risk of bias. Furthermore, funnel plots and Egger's test were carried out to assess publication bias; P values >.1 were considered as indicative of no publication bias.
2.4. Data synthesis and analysis
Statistical analyses were performed using Review Manager 5.3 (RevMan 5.3) (The Cochrane Collaboration, Copenhagen, Denmark). Measures of association such as hazard ratio (HR) and relative risk (RR) were converted into odds ratio (OR), which was the only association measure used. 30 , 31 OR, HR and RR adjusted were included in the analysis as they were reported. In order to analyze continuous NLR values, we used the Chinn's method. 32 This method allowed us to transform standardised mean differences to their equivalent OR per study. Then we calculated the natural logarithm of the OR (logOR) and its standard error (SE[logOR]) for each one of the studies. The variables reported as medians and interquartile ranges (IQRs) were converted into means and standard deviations (SD), respectively. The mean was estimated by the formula x = (a + 2m + b)/4 using the values of the median (m), P25 and P75 (a and b, respectively). Likewise, the SD was estimated using the following formula: SD = IQR/1.35. 33 , 34
The heterogeneity of the studies in the measure of the effects was evaluated using the I 2 statistics. Values greater than 60% were considered as severe heterogeneity, 40%‐60% as moderate heterogeneity and less than 40% as mild heterogeneity. The Cochran Q test was also reported. A P value of <.05 was considered statistically significant. We conducted a random effects meta‐analyses as we anticipated that there was heterogeneity among studies. We performed subgroup analyses by location of the study (Chinese vs non‐Chinese studies) and study design (cohorts, case‐control studies) and reported the interaction test P value per subgroup analysis. Finally, sensitivity analyses were performed only using the low risk of bias studies.
3. RESULTS
3.1. Study selection
The flow diagram summarising the process of study retrieval is shown in Figure 1. In the initial electronic search, a total of 925 records were found. After excluding duplicate studies, 483 studies were preserved. Subsequently, during the evaluation of titles and abstracts, 365 more records were excluded. Finally, during the full‐text assessment, 57 articles were excluded as a result of group imbalance, outcome not reported, wrong population, or the patients were not older than 18 years. Finally, 61 studies were selected for the qualitative and quantitative syntheses.
FIGURE 1.
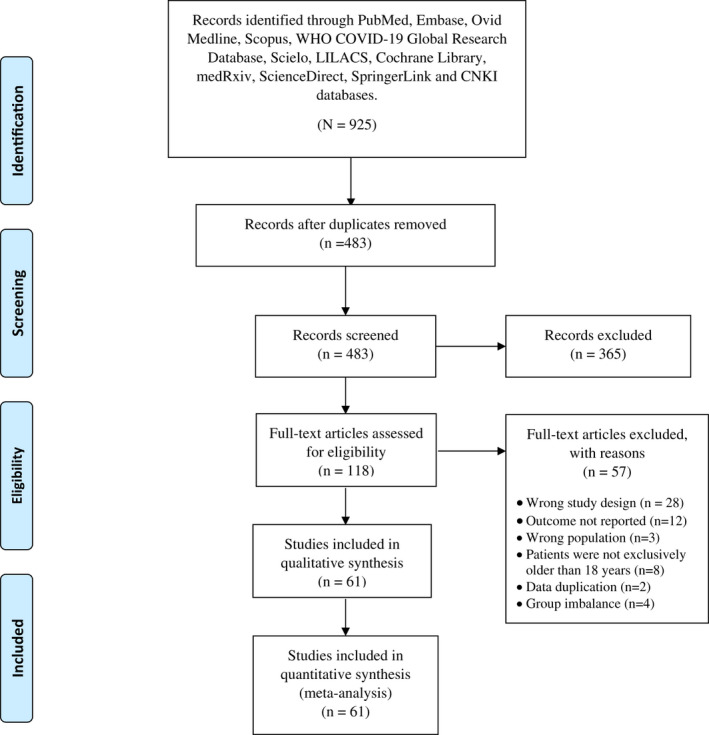
PRISMA 2009 flow diagram
3.2. Study characteristics
The characteristics of the studies are presented in Table 1, 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 and in Supporting InformationTable S1. 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 For this systematic review, 58 cohort studies and three case‐control studies were included, most of them conducted in China and 20 studies in other countries. On the other hand, our primary outcome (severity) was present in 36 studies, 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 the secondary outcome (mortality) was present in 28 studies, 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 and three studies analysed both outcomes. 62 , 71 , 72
TABLE 1.
Characteristics of studies evaluating the association of NLR and severity
| Author | Year | Participants (male) | Median/mean age (IQR/SD) | NLR description | Type of outcome | NLR mean (SD) in severe patients | NLR mean (SD) in nonsevere patients | SD mean difference between severe and nonsevere patients | OR (adjusted) | HR (adjusted) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chuan Qin et al | 2020 | 452 (235) | 57.5 (14.81) | Quantitative | Severity | 6.1 (4.96) | 3.27 (2.3) | 0.67 [0.48, 0.87] | NR | NR |
| Xiurong Ding et al | 2020 | 72 (33) | 49 0.75 (20) | Quantitative | Severity | 4.8 (5.33) | 2 (1.18) | 1.06 [0.47, 1.66] | NR | NR |
| Yafei Zhang e al. | 2020 | 115 (49) | 49.52 (17.06) | Quantitative | Severity | 758 (7.04) | 2.28 (1.29) | 1.39 [0.94, 1.74] | NR | NR |
| Fengjun Liu et al | 2020 | 134 (63) | 51.25 (20.74) | Quantitative | Severity | 3.85 (2.22) | 2.72 (1.41) | 0.73 [0.23, 1.22] | NR | NR |
| Xiaomin Luo et al | 2020 | 298 (150) | 55.75 (21.48) | Quantitative | Severity | 6.28 (4.17) | 2.68 (1.32) | 1.47 [1.02, 1.93] | NR | NR |
| Mortality | ||||||||||
| Ruchong Chen et al | 2020 | 548 (313) | 56 (14.5) | Quantitative | Severity | 9.89 (9.2) | 3.86 (3.4) | 1.03 [0.83, 1.23] | NR | NR |
| Mortality | ||||||||||
| Hou Keke et al | 2020 | 56 (29) | 48 (13.5) | Quantitative | Severity | 6.13 (6.08) | 4.01 (5.62) | 0.36 [−0.31, 1.04] | NR | NR |
| Changzheng Wang et al | 2020 | 45 (23) | 39 (34.07) | Quantitative | Severity | 29.9 (18.7) | 7.93 (8.36) | 1.90 [1.09, 2.72] | NR | NR |
| Jianhong Fu et al | 2020 | 75 (45) | 46.6 (14) | Quantitative | Severity | 6.29 (3.72) | 2.3 (1.22) | 1.97 [1.33, 2.61] | NR | NR |
| Song CY et al | 2020 | 79 (49) | 54 (45‐63) | Quantitative | Severity | 9.87 (11.3) | 3.35 (2.6) | 0.72 [0.25, 1.19] | NR | NR |
| Lian J. et al | 2020 | 203 (90) | 66 (62‐71) | Quantitative | Severity | 4.18 (2.82) | 2.59 (1.35) | 0.82 [0.51, 1.13] | NR | NR |
| Gormez S et al | 2020 | 247 (154) | 51.3 (14.2) | Quantitative | Severity | 8.13 (5.82) | 3.18 (2.33) | 1.50 [1.15, 1.84] | NR | NR |
| Feng Z et al | 2020 | 141 (72) | 44 (34‐55) | Quantitative | Severity | 4.45 (1.48) | 2.55 (1.18) | 1.56 [0.99, 2.12] | NR | NR |
| Bennouar S et al | 2020 | 330 (206) | 66.6 (8.9) | Quantitative | Severity | 12.7 (10.9) | 5.1 (4.4) | 0.96 [0.73, 1.19] | NR | NR |
| Qun S et al | 2020 | 225 (96) | 59.8 (14) | Quantitative | Severity | 2.96 (2.47) | 2.41 (1.39) | 0.34 [−0.02, 0.70] | NR | NR |
| Xue G et al | 2020 | 114 (64) | 62 (51‐70) | Quantitative | Severity | 6.58 (4.91) | 3.075 (1.86) | 0.93 [0.54, 1.32] | NR | NR |
| Zhichao F et al | 2020 | 141 (72) | 44 (34‐45) | Quantitative | Severity | 5.1 (2.81) | 3.15 (1.48) | 1.17 [0.61, 1.72] | NR | NR |
| Chen et al | 2020 | 132 (76) | 63.4 (56‐71) | Quantitative | Severity | 7.63 (6.97) | 4.475 (3.9) | 0.69 [0.23, 1.14] | NR | NR |
| Mortality | ||||||||||
| Ok F et al | 2020 | 139 (62) | 55.5 (18.5) | Quantitative | Severity | 6.1 (5.1) | 2.46 (2.3) | 0.99 [0.63, 1.35] | NR | NR |
| Basbus L et al | 2020 | 131 (71) | 52 (36‐77) | Qualitative | Severity | NR | NR | NR | 8.73 (2.73‐27.85) a | NR |
| Cheng B et al | 2020 | 456 (211) | 54.97 (18.59) | Quantitative | Severity | 3.615 (2.6) | 2.16 (1.35) | 0.68 [0.49, 0.87] | NR | NR |
| Shi S et al | 2020 | 87 (49) | 60 (22‐88) | Quantitative | Severity | 7.3 (5.97) | 2.2 (0.97) | 1.30 [0.83, 1.77] | NR | NR |
| Asan A et al | 2020 | 695 (331) | NR | Quantitative | Severity | 6.6 (7.8) | 2.4 (2) | 1.69 [1.30, 2.09] | NR | NR |
| Lei Liu et al | 2020 | 294 (162) | 56.0 (39‐67) | Quantitative | Severity | 12.33 (10.45) | 2.85 (2.07) | 1.55 [1.28, 1.83] | NR | NR |
| Hu Haifeng et al | 2020 | 40 (24) | 51.0 (42.0‐66.8) | Quatitative | Severity | 10.59 (12.33) | 3.13 (2.4) | 0.80 [0.16, 1.45] | NR | NR |
| Güner R et al | 2020 | 222 (132) | 50.6 (16.5) | Quantitative | Severity | 12.7 (27) | 8.35 (20.4) | 0.20 [−0.12, 0.51] | NR | NR |
| Gong J et al | 2020 | 189 (88) | 49 (35‐63) | Quantitative | Severity | 4.03 (3.48) | 2.03 (1.11) | 1.19 [0.77, 1.61] | NR | NR |
| Liao D et al | 2020 | 294 (145) | NR | Quantitative | Severity | 4.96 (3.82) | 2.78 (1.78) | 0.73 [0.50, 0.97] | NR | NR |
| Ai‐ping Yang et al | 2020 | 93 (56) | 46.4 (17.6) | NLR < 3 | Severity | 20.7 (24.1) | 4.8 (3.5) | 1.26 [0.76, 1.76] | NR | NR |
| NLR ≥ 3 | ||||||||||
| Weifeng Shang et al | 2020 | 443 (220) | 55.475 (17.4) | NLR ≥ 4.283 | Severity | 5.36 (5.11) | 2.51 (1.59) | 0.9 [0.69, 1.11] | NR | NR |
| NLR < 4.283 | ||||||||||
| Chen Xi et al | 2020 | 139 (76) | 45.5 (13.3) | NLR < 4.5 | Severity | 4.47 (2.99) | 3.31 (1.92) | 0.52 [0.12, 0.93] | NR | NR |
| NLR ≥ 4.5 | ||||||||||
| Xintian Xia et al | 2020 | 63 (33) | NR | NLR < 4.795 | Severity | 12.1 (14.32) | 5.77 (10.2) | 0.50 [0.00, 1.01] | NR | NR |
| NLR > 4.795 | ||||||||||
| Li Long et al | 2020 | 301 (150) | 50.25 (20) | NLR < 2.973 | Severity | NR | NR | NR | NR | 2.641 (1.421‐4.908) P = 0.002* |
| NLR ≥ 2.973 | ||||||||||
| Yue‐Ping Liu et al | 2020 | 84 (47) | 54.25 (52.59) | NLR < 4.87 | Severity | 19.75 (48.96) | 4.3 (7.88) | 0.58 [0.10, 1.07] | NR | NR |
| NLR ≥ 4.87 | ||||||||||
| Suyu Sun et al | 2020 | 116 (60) | 49.5 (11.85) | NLR < 4.5 | Severity | 8.9 (7.9) | 2.5 (1.28) | 1.61 [1.14, 2.09] | NR | NR |
| NLR ≥ 4.5 | ||||||||||
| Chen Xing et al | 2020 | 296 (137) | NR | NR | Severity | 3.86 (3.28) | 1.88 (1.03) | 1.39 [1.00, 1.78] | NR | NR |
Abbreviations: HR, hazard ratio; IQR, interquartile range; NR, not reported NLR, neutrophil‐to‐lymphocyte ratio; OR, odds ratio; SD, standard deviation.
Adjusted to age and hypertension.
Adjusted to sex, age, comorbidities, eosinophil count and C‐reactive protein level.
There was a total of 15 522 patients within the studies, 53.74% were men and age ranged from 22 to 81 years. Seven studies did not present information about age. In 11 studies, the days elapsed for the development of severity, from the day of admission, were reported, whose average was 5.64 days and ranged from 4 to 14 days.
The NOS was used for the quality assessment of the studies (see Supporting Information Table S2). It was identified that 2 studies had a high risk of bias, 21 studies had a moderate risk of bias and only 38 had a low risk of bias.
3.3. Association of NLR with disease severity in hospitalised COVID‐19 patients
This association was evaluated in 36 studies (n = 7489). As shown in Figure 2A, we found that higher NLR levels were associated with higher odds of severity in patients with hospitalised COVID‐19 diagnosis (OR 6.09; 95% CI 4.82 to 7.71; P < .001). Because of severe heterogeneity (I 2 = 79%, P < .001), subgroup analysis by study design (Figure 2B) did not change the main effects (cohorts: OR 6.33; 95% CI 4.96 to 8.06; P < .001 vs case‐control studies: OR 3.05; 95% CI 1.64 to 5.68; P = .53; interaction test P = .03). Likewise, the subgroup analysis by country of origin (Figure 2C) showed differences between Chinese (OR 5.9; 95% CI 4.63 to 7.53; P < .001) and non‐Chinese studies (OR 7.03; 95% CI 3.22 to 15.33; P < .001, interaction test P < .68). In sensitivity analysis, which included only studies at low risk of bias, the association between NLR values and severity was still present (OR 5.17; 95% CI 4.31 to 6.2; P < .001) with moderate heterogeneity (I 2 = 53%, P < .001) (Figure 2D).
FIGURE 2.
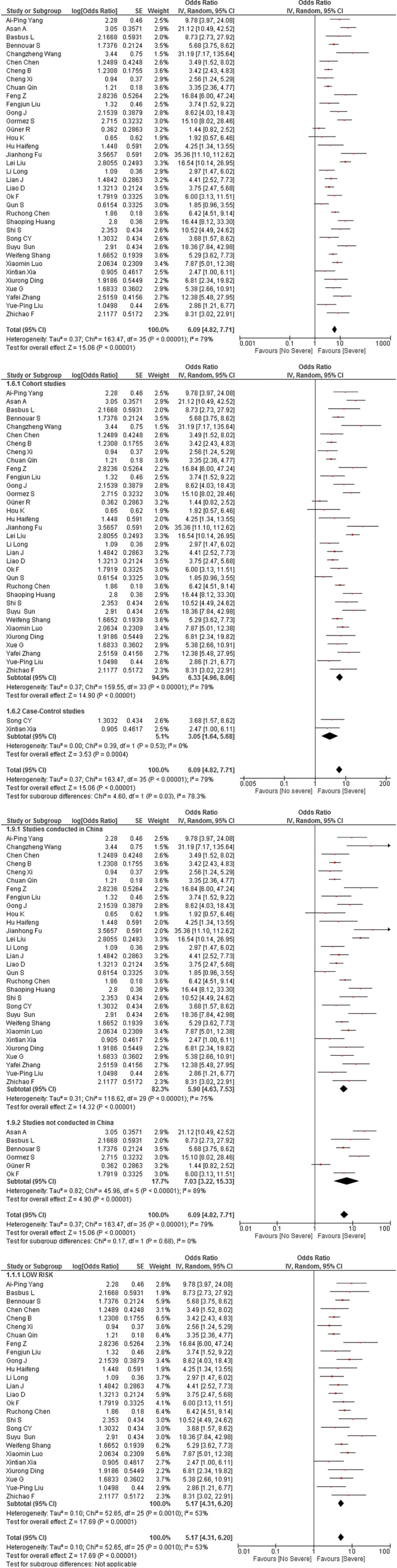
(A) Association of NLR and COVID‐19 severity. (B) Subgroup analysis according to study design of the association between NLR and severity in COVID‐19 patients. (C) Subgroup analysis according to the origin country of the association between NLR and severity in COVID‐19 patients. (D) Sensitivity analysis according to risk of bias of the association between NLR and severity in COVID‐19 patients
3.4. Association of NLR with all‐cause mortality in hospitalised COVID‐19 patients
This association was evaluated in 28 studies (n = 8033). As presented in Figure 3A, we found that higher values of NLR were associated with higher odds of all‐cause mortality in hospitalised COVID‐19 patients (OR 12.6; 95% CI 6.88 to 23.06; P < .001) with high heterogeneity of effects (I 2 = 98%). The subgroup analysis by country of origin (Figure 3B) showed that the strength of the association between NLR and mortality was even higher in Chinese studies (OR 26.94; 95% CI 14.57 to 49.81; P < .001) with high heterogeneity (I 2 = 92%); whereas the association in the non‐Chinese studies was very different compared with the main mortality analysis (OR 5.89 95% CI 3.18 to 10.9; P < .001). There were differences between effects according to country of origin (interaction test P < .001). Regarding the subgroup analysis by study design (Figure 3C), both cohort (OR 12.51 95% CI 6.73 to 23.27; P < .001) and case‐control (OR 15.1 95% CI 9.07 to 25.14; P < .001) studies revealed higher odds of mortality (interaction test P = .65). In the sensitivity analysis of low risk of bias studies, there was moderate heterogeneity (OR 10.42 95% CI 7.73 to 14.06; P = .005; I 2 = 58%, χ2 P = .005) (Figure 3D).
FIGURE 3.
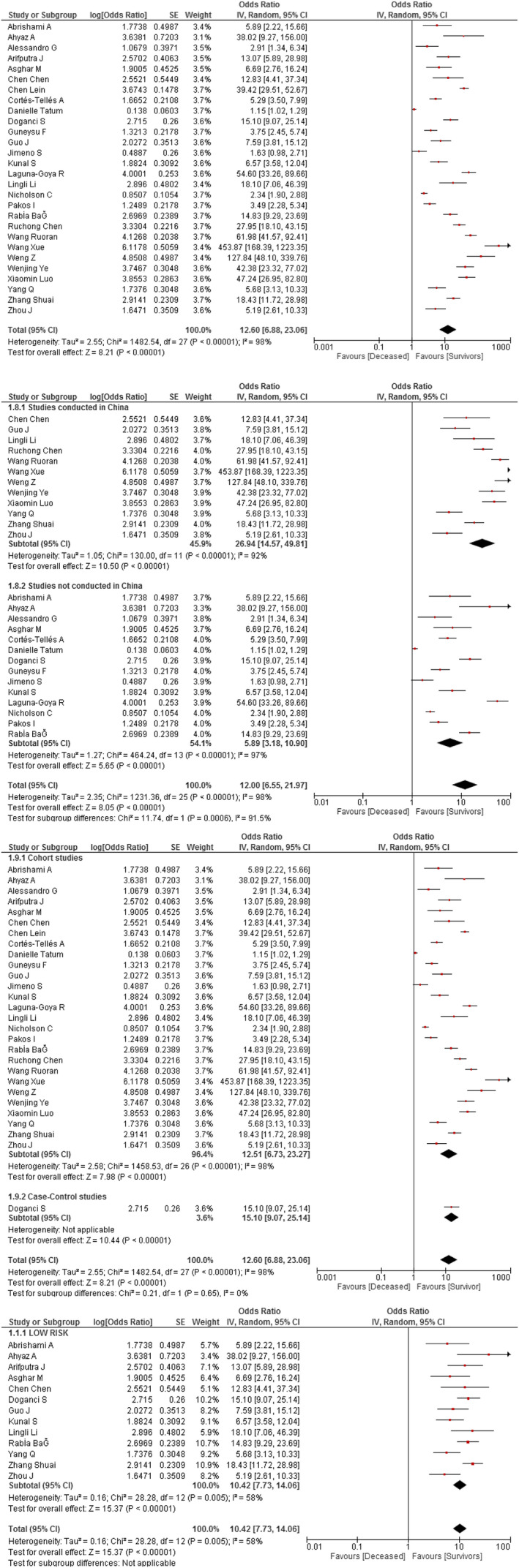
(A) Association between NLR and mortality in COVID‐19 patients. (B) Subgroup analysis according to the origin country of the association between NLR and mortality in COVID‐19 patients. (C) Subgroup analysis according to study design of the association between NLR and mortality in COVID‐19 patients. (D) Sensitivity analysis according to risk of bias of the association between NLR and mortality in COVID‐19 patients
3.5. Publication bias
There was no indication that there were small study effects for the severity of disease (Egger's test P = .112) (see Supporting Information Figures S4.A and S4.B).
4. DISCUSSION
In the current context of the COVID‐19 pandemic, an efficient, fast and cheap method is required to determine the prognosis of patients with COVID‐19. Given the growing number of studies that established NLR as a possible prognostic biomarker of severity and mortality in patients diagnosed with COVID‐19, we decided to carry out a systematic review and a meta‐analysis to consolidate the information regarding this topic. The present meta‐analysis incorporated a total of 61 studies and found that high NLR values on admission day were associated with progression towards severity and mortality.
The prognostic value of NLR has been studied and correlated to multiple chronic, inflammatory and infectious diseases, 14 , 15 , 16 , 17 , 18 , 19 , 20 such as community‐acquired pneumonia (CAP), where NLR had a more significant prognostic performance towards severity than other markers such as white blood cell count, C‐reactive protein, and neutrophil count. 96 Likewise, NLR has also been proven to predict 30‐day mortality in CAP with a positive predictive value of 100% and a negative predictive value of 78%. 97
The hemogram is usually altered in COVID‐19 patients, being higher in patients with severe illness compared with mild illness. 98 This could be reflected in the cohort study conducted by Wang S. et al in COVID‐19 patients where it was found that an increase on NLR values was associated with severity (OR 8.56, 95% CI, 1.39‐52.61, P = .021) as we found in our study. 99 The biological mechanism by which these variations arise in the neutrophil and lymphocyte counts has not been elucidated so far; however, several possible explanations have been proposed. The first one is based on the physiological relationship that exists between systemic inflammation and stress with the appearance of neutrophilia and lymphocytopaenia. The second possible explanation is based on the depletion of the number of lymphocytes, especially CD4 + and CD8 + T cells. These two agents have, as one of their functions, the regulation of the immune system response against viral infections. A low circulating number of these two lymphocytes could cause a generalised dysregulation of the immune system, especially of neutrophils. On the other hand, lymphocytopaenia has been linked to lymphocyte exhaustion and to the ability of SARS‐CoV‐2 to infect lymphocytes. Lymphocyte exhaustion occurs in chronic inflammatory processes where there is a continuous and excessive stimulation of T lymphocytes that causes their exhaustion and therefore impairing their functions. 100 , 101 , 102 , 103 All in all, several of the latest prediction scores include NLR as part of their prognostic variables. 104
Two meta‐analyses have previously been published where the prognostic value of NLR was analysed in patients diagnosed with COVID‐19, the first one by Lagunas‐Rangel 105 and the second one by Xudong Feng et al. 25 Despite the existence of these studies, it was necessary to carry out a systematic review exclusively about the neutrophil‐lymphocyte ratio because the previous studies presented an exceedingly small number of studies incorporated in the meta‐analysis (only five and six studies, respectively). Moreover, they used few databases for the literature search, and they did not perform the sensitivity analysis, which allows identifying possible sources of heterogeneity. Specifically, in the article done by Lagunas‐Rangel, a heterogeneity of 96, 45% was reported, and despite this, it was concluded that there was an association between the NLR and the progression to severity. This is an error since high variability suggests that studies should not be combined in a meta‐analysis.
Our meta‐analysis contribution was to perform a conversion from the mean difference to a more reliable measure of effect, such as OR through Chinn's method. 32 This conversion allowed us to include those studies that have no continuous values for NLR. In our sensibility analysis, the moderate/high risk of bias studies was possibly the primary source of heterogeneity. It is important to emphasise this last point because the desire to produce scientific knowledge that helps guide therapeutic decisions during the pandemic has caused studies to be carried out in an expeditious manner, often by personnel with little methodological knowledge and without adequate advice. 106 This has resulted in a low‐quality scientific production that has been reflected in the present study since 23 of the 61 studies analysed have a moderate to high risk of bias.
4.1. Limitations
Our study has several limitations. First, our meta‐analysis reported high OR values and broad CI for both outcomes. This could be because of some small sample sizes and clinical diversity. When we did the conversion, the values of the standardised mean differences, which we use for the OR conversion, were very high, so that also influences the high OR values. The broad CI could be explained by some small sample sizes, so the effect is detected but has low precision. Second, all the incorporated studies in this systemic review, except for one, were developed in China, which do not allow a fair ethnic comparison in COVID‐19 patients. Third, we found high heterogeneity between the included studies, which was traced back to the bad quality found in some publications. Finally, there was no consensus among the articles analysed regarding the cutoff to define elevated NLR and the severity definition differed between some studies that could lead to bias.
5. CONCLUSIONS
In the presented systematic review and meta‐analysis, the elevated NLR values were clearly associated with the development of severity and mortality in patients diagnosed with COVID‐19. Therefore, an elevated NLR could be used as an early and easy prognostic parameter for severity and mortality in COVID‐19 patients.
DISCLOSURES
Authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study design and concept: IST, JRUB, JLM, AVH and VB‐Z. Acquisition of data: AAC, EA‐B, JRUB, IST, AVH, VB‐Z and JLM. Drafting of the manuscript: JRUB, IST, AAC and EA‐B. Critical revision of the manuscript: AVH, VB‐ZM and JLM. Statistical analysis: VB‐ZM, JRUB, IST and AVH. Study supervision: AVH, JLM and VB‐Z.
Supporting information
Supplementary Material
Ulloque‐Badaracco JR, Ivan Salas‐Tello W, Al‐kassab‐Córdova A, et al. Prognostic value of neutrophil‐to‐lymphocyte ratio in COVID‐19 patients: A systematic review and meta‐analysis. Int J Clin Pract. 2021;75:e14596. 10.1111/ijcp.14596
DATA AVAILABILITY STATEMENT
Data available on request from the authors—The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID‐19: their roles in pathogenesis. J Microbiol Immunol Infect. 2021;54:159‐163. 10.1016/j.jmii.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahn D‐G, Shin H‐J, Kim M‐H, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID‐19). J Microbiol Biotechnol. 2020;30:313‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Graham Carlos W, Dela Cruz CS, Cao B, Pasnick S, Jamil S. Novel Wuhan (2019‐nCoV) coronavirus. Am J Respir Crit Care Med. 2020;201:P7‐P8. 10.1164/rccm.2014P7 [DOI] [PubMed] [Google Scholar]
- 4. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—an update on the status. Military Medical Research. 2020;7: 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO announces COVID‐19 outbreak a pandemic . World Health Organization; 2020.
- 6.Coronavirus disease (COVID‐19) Situation Report‐137 Highlights situation in numbers (by WHO Region). 2021.
- 7. Alimohamadi Y, Taghdir M, Sepandi M. Estimate of the basic reproduction number for COVID‐19: a systematic review and meta‐analysis. J Prev Med Public Heal. 2020;53:151‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Priyadarshini I, Mohanty P, Kumar R, et al. Analysis of outbreak and global impacts of the COVID‐19. Healthcare. 2020;8:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID‐19 patients: a systematic review and meta‐analysis. J Med Virol. 2020;92:1875‐1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mehraeen E, Karimi A, Barzegary A, et al. Predictors of mortality in patients with COVID‐19–a systematic review. Eur J Integr Med. 2020;40:101226–. 10.1016/j.eujim.2020.101226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Al‐Tawfiq JA, Leonardi R, Fasoli G, Rigamonti D. Prevalence and fatality rates of COVID‐19: What are the reasons for the wide variations worldwide? Travel Med Infect Dis. 2020;35:101711. 10.1016/j.tmaid.2020.101711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. He W, Yi GY, Zhu Y. Estimation of the basic reproduction number, average incubation time, asymptomatic infection rate, and case fatality rate for COVID‐19: meta‐analysis and sensitivity analysis. J Med Virol. 2020;jmv.26041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quah P, Li A, Phua J. Mortality rates of patients with COVID‐19 in the intensive care unit: a systematic review of the emerging literature. Crit Care. 2020;24:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dong CH, Wang ZM, Chen SY. Neutrophil to lymphocyte ratio predict mortality and major adverse cardiac events in acute coronary syndrome: a systematic review and meta‐analysis. Clin Biochem. 2018;52:131‐136. [DOI] [PubMed] [Google Scholar]
- 15. Luo X, Zhou L. Prognostic significance of neutrophil to lymphocyte ratio in patients with gastrointestinal stromal tumors: a meta‐analysis. Clinica Chimica Acta. Elsevier B.V. 2018;477:7‐12. [DOI] [PubMed] [Google Scholar]
- 16. Jin J, Yang L, Liu D, Li W. Association of the neutrophil to lymphocyte ratio and clinical outcomes in patients with lung cancer receiving immunotherapy: a meta‐analysis. BMJ Open. 2020;10(6):e035031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen G, Zhu L, Yang Y, Long Y, Li X, Wang Y. Prognostic role of neutrophil to lymphocyte ratio in ovarian cancer: a meta‐analysis. Technol Cancer Res Treatment. 2018;17:153303381879150. 10.1177/1533033818791500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurtul BE, Ozer PA. Neutrophil‐to‐lymphocyte ratio in ocular diseases: a systematic review. Int J Ophthalmol. 2019;12:1951‐1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peng Y Li Y, He Y, et al. The role of neutrophil to lymphocyte ratio for the assessment of liver fibrosis and cirrhosis: a systematic review. Expert Rev Gastroenterol Hepatol. 2018;12:503‐513. 10.1080/17474124.2018.1463158 [DOI] [PubMed] [Google Scholar]
- 20. Russell CD, Parajuli A, Gale HJ, et al. The utility of peripheral blood leucocyte ratios as biomarkers in infectious diseases: a systematic review and meta‐analysis. J Infect. 2019;78:339‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zahorec R. Ratio of neutrophil to lymphocyte counts‐rapid and simple parameter of systemic inflammation and stress in critically ill. BRATISL LEK List. 2001;102:5‐14. [PubMed] [Google Scholar]
- 22. Forget P, Khalifa C, Defour J‐P, Latinne D, Van Pel M‐C, De Kock M. What is the normal value of the neutrophil‐to‐lymphocyte ratio? BMC Res Notes. 2017;10(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moosazadeh M, Maleki I, Alizadeh‐Navaei R, et al. Normal values of neutrophil‐to‐lymphocyte ratio, lymphocyte‐to‐monocyte ratio and platelet‐to‐lymphocyte ratio among Iranian population: results of Tabari cohort. Casp J Intern Med. 2019;10:320‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kermali M, Khalsa RK, Pillai K, Ismail Z, Harky A. The role of biomarkers in diagnosis of COVID‐19—A systematic review. Life Sci. 2020;254:117788. 10.1016/j.lfs.2020.117788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feng X, Li S, Sun Q, et al. Immune‐inflammatory parameters in COVID‐19 cases: a systematic review and meta‐analysis. Front Med. 2020;7:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40‐46. [DOI] [PubMed] [Google Scholar]
- 28. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(210). 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wells G, Shea B, O’Connell D, et al. The Newcastle‐Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses; 2011.
- 30. Shor E, Roelfs D, Vang ZM. The “Hispanic mortality paradox” revisited: meta‐analysis and meta‐regression of life‐course differentials in Latin American and Caribbean immigrants' mortality. Soc Sci Med. 2017;186:20‐33. [DOI] [PubMed] [Google Scholar]
- 31. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. J Am Med Assoc. 1998;280:1690‐1691. [DOI] [PubMed] [Google Scholar]
- 32. Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Stat Med. 2000;19(22):3127‐3131. [DOI] [PubMed] [Google Scholar]
- 33. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (Eds.). Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID‐19) in Wuhan, China. Clin Infect Dis. 2020;71:762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ding X, Yu Y, Lu B, et al. Dynamic profile and clinical implications of hematological parameters in hospitalized patients with coronavirus disease 2019. Clin Chem Lab Med. 2020;8:1365‐1371. [DOI] [PubMed] [Google Scholar]
- 37. Chen X, Tong J, Xiang J, Hu J. Retrospective study on the epidemiological characteristics of 139 patients with novel coronavirus pneumonia on the effects of severity. Chongqing Medicine. 2020;49:2802–2806. https://doi.org/10.3969/j.issn.1671‐8348.2020.14.001 [Google Scholar]
- 38. Xia X, Wen M, Zhan S, He J, Chen W. An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID‐19. Nan Fang Yi Ke Da Xue Xue Bao. 2020;40:333‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Long L, Zeng X, Zhang X, et al. Short‐term outcomes of COVID‐19 and risk factors for progression. Eur Respir J. 2020;318:50‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y‐P, Li G‐M, He J, et al. Combined use of the neutrophil‐to‐lymphocyte ratio and CRP to predict 7‐day disease severity in 84 hospitalized patients with COVID‐19 pneumonia: a retrospective cohort study. Ann Transl Med. 2020;8:635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID‐19 in Wenzhou, China. Clin Chim Acta. 2020;507:174–180. 10.1016/j.cca.2020.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xing C, Jingyi OU, Tan Y, et al. Diagnostic roles of several parameters in coronavirus disease 2019. Lab Med. 2020;35:295‐299. 10.3969/j.issn.1673-8640.2020.04.002. [DOI] [Google Scholar]
- 43. Gormez S, Ekicibasi E, Degirmencioglu A, et al. Association between renin–angiotensin–aldosterone system inhibitor treatment, neutrophil–lymphocyte ratio, D‐Dimer and clinical severity of COVID‐19 in hospitalized patients: a multicenter, observational study. J Hum Hypertens. 2021. 10.1038/s41371-020-00405-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liao D Zhou F, Luo L, et al. Haematological characteristics and risk factors in the classification and prognosis evaluation of COVID‐19: a retrospective cohort study. Lancet Haematol. 2020;7:e671‐e678. 10.1016/S2352-3026(20)30217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang H, Wang LL, Chen YY, et al. A tool to early predict severe corona virus disease 2019 (COVID‐19) : a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Cancer. 2020;46:1–17. 10.1007/s00134-020-06023-4%0A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Güner R, Hasanoğlu İ, Kayaaslan B, et al. COVID‐19 experience of the major pandemic response center in the capital: results of the pandemic's first month in Turkey. Turkish J Med Sci. 2020;50:1801‐1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang Y, Zheng L, Liu L, Zhao M, Xiao J, Zhao Q. Liver impairment in COVID‐19 patients: a retrospective analysis of 115 cases from a single centre in Wuhan City, China. Liver Int. 2020;40:2095‐2103. [DOI] [PubMed] [Google Scholar]
- 48. Xue G, Gan X, Wu Z, et al. Novel serological biomarkers for inflammation in predicting disease severity in patients with COVID‐19. Int Immunopharmacol. 2020;89:107065. 10.1016/j.intimp.2020.107065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Qun S, Wang Y, Chen J, et al. Neutrophil‐to‐lymphocyte ratios are closely associated with the severity and course of non‐mild COVID‐19. Front Immunol. 2020;11:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bennouar S, Bachir Cherif A, Kessira A, et al. Usefulness of biological markers in the early prediction of corona virus disease‐2019 severity. Scand J Clin Lab Invest. 2020;80:611‐618. 10.1080/00365513.2020.1821396 [DOI] [PubMed] [Google Scholar]
- 51. Feng Z, Yu Q, Yao S, Luo L, Duan JYZ. Early prediction of disease progression in 2019 novel coronavirus pneumonia patients outside Wuhan with CT and clinical characteristic. MedRxiv. 2020. [Google Scholar]
- 52. Lian J, jin C, Hao S, et al. High neutrophil‐to‐lymphocyte ratio associated with progression to critical illness in older patients with COVID‐19: a multicenter retrospective study. Aging (Albany NY). 2020;12:13849‐13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hu H, Du H, Li J, et al. Early prediction and identification for severe patients during the pandemic of COVID‐19: a severe COVID‐19 risk model constructed by multivariate logistic regression analysis. J Glob Health. 2020;10:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Basbus L, Lapidus MI, Martingano I, Puga MC, Pollán J. Índice neutrófilo‐linfocito como factor pronóstico de covid‐19. Med (Buenos Aires). 2020;80:31‐36. [PubMed] [Google Scholar]
- 55. Liu L, Zheng Y, Cai L, et al. Neutrophil‐to‐lymphocyte ratio, a critical predictor for assessment of disease severity in patients with COVID‐19. Int J Lab Hematol. 2021;43:329‐335. [DOI] [PubMed] [Google Scholar]
- 56. Ok F, Erdogan O, Durmus E, Carkci S, Canik A. Predictive values of blood urea nitrogen/creatinine ratio and other routine blood parameters on disease severity and survival of COVID‐19 patients. J Med Virol. 2021;93:786‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cheng B, Hu J, Zuo X, et al. Predictors of progression from moderate to severe coronavirus disease 2019: a retrospective cohort. Clin Microbiol Infect. 2020;26:1400‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu F, Zhang QI, Huang C, et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID‐19 patients. Theranostics. 2020;10:5613‐5622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Asan A, Üstündağ Y, Koca N, et al. Do initial hematologic indices predict the severity of COVID‐19 patients? Turkish J Med Sci. 2021;51:39‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shi SI, Liu X, Xiao J, et al. Prediction of adverse clinical outcomes in patients with coronavirus disease 2019. J Clin Lab Anal. 2021;35:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Song CY, Xu J, He JQ, Lu YQ. Immune dysfunction following COVID‐19, especially in severe patients. Sci Rep. 2020;10:1‐11. 10.1038/s41598-020-72718-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen C, Zhang J, Li C, et al. The characteristics and death risk factors of 132 COVID‐19 pneumonia patients with comorbidities: a retrospective single center analysis in Wuhan, China. medRxiv. 2020. [Google Scholar]
- 63. Wang C, Deng R, Gou L, et al. Preliminary study to identify severe from moderate cases of COVID‐19 using combined hematology parameters. Ann Transl Med. 2020;8:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hou KK, Zhang N, Zhou M. CT features of corona virus disease 2019 (COVID‐19) in different stages and its correlation with neutrophil‐lymphocyte ratio (NLR) and T lymphocyte subsets. Radiol Practice. 2020;35:272‐276. https://10.13609/j.cnki.1000‐0313.2020.03.006. [Google Scholar]
- 65. Fu J, Kong J, Wang W, et al. The clinical implication of dynamic neutrophil to lymphocyte ratio and D‐dimer in COVID‐19: a retrospective study in Suzhou China. Thromb Res. 2020;19:3–8. 10.1016/j.thromres.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huang S, Liu M, Li X, Shang Z, Zhang T, Lu H. Significance of neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio for predicting clinical outcomes in COVID‐19. MedRxiv. 2020;386. 10.1101/2020.05.04.20090431. [DOI] [Google Scholar]
- 67. Yang A, Liu J, Tao W, Li H. The diagnostic and predictive role of NLR, d‐NLR and PLR in COVID‐19 patients. Int Immunopharmacol. 2020;84:106504. 10.1016/j.intimp.2020.106504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shang W, Dong J, Ren Y, et al. The value of clinical parameters in predicting the severity of COVID‐19. J Med Virol. 2020;92:2188‐2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li L, Yang L, Gui S, et al. Association of clinical and radiographic findings with the outcomes of 93 patients with COVID‐19 in Wuhan, China. Theranostics. 2020;10:6113‐6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Tatum D, Taghavi S, Houghton A, Stover J, Toraih E, Duchesne J. Neutrophil‐to‐lymphocyte ratio and outcomes in Louisiana COVID‐19 patients. Shock. 2020;54:652‐658. 10.1097/SHK.0000000000001585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Luo X, Zhou W, Yan X, et al. Prognostic Value of C‐Reactive Protein in Patients With Coronavirus 2019. Clin Infect Dis. 2020;71 :2174–2179. 10.1093/cid/ciaa641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID‐19 patients in China. J Allergy Clin Immunol. 2020;146:89‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Asghar MS, Haider Kazmi SJ, Ahmed Khan N, et al. Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID‐19 patients in Pakistan: a single‐center retrospective study in a tertiary care hospital of Karachi correction. Cureus. 2020;12. 10.7759/cureus.c34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bağ soytaş R, Ünal D, Arman P, et al. Factors affecting mortality in geriatric patients hospitalized with COVID‐19. Turkish J Med Sci. 2021;454‐463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arifputra J, Waleleng BJ, Gosal F, et al. Liver transaminase levels and neutrophil to lymphocyte ratio as prognostic and predictor in coronavirus disease 2019. Open Access Maced J Med Sci. 2020;8:282‐285. [Google Scholar]
- 76. Chen L, Yu J, He W, et al. Risk factors for death in 1859 subjects with COVID‐19. Leukemia. 2020;34:2173‐2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Nicholson CJ, Wooster L, Sigurslid HH, et al. Estimating risk of mechanical ventilation and in‐hospital mortality among adult COVID‐19 patients admitted to Mass General Brigham: the VICE and DICE scores. EClinicalMedicine. 2021;33:100765 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fois AG, Paliogiannis P, Scano V, et al. The systemic inflammation index on admission predicts in‐hospital mortality in COVID‐19 patients. Molecules. 2020;25:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhou J, Huang L, Chen J, et al. Clinical features predicting mortality risk in older patients with COVID‐19. Curr Med Res Opin. 2020;36:1753‐1759. 10.1080/03007995.2020.1825365 [DOI] [PubMed] [Google Scholar]
- 80. Ayaz A, Arshad A, Malik H, Ali H, Hussain E, Jamil B. Risk factors for intensive care unit admission and mortality in hospitalized COVID‐19 patients. Acute Crit Care. 2020;35:249‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jimeno S, Ventura PS, Castellano JM, et al. Prognostic implications of neutrophil‐lymphocyte ratio in COVID‐19. Eur J Clin Invest. 2021;51:1‐9. [DOI] [PubMed] [Google Scholar]
- 82. Wang X, Li X, Shang Y, et al. Ratios of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte predict all‐cause mortality in inpatients with coronavirus disease 2019 (COVID‐19): a retrospective cohort study in a single medical center. Epidemiol Infect. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ye W, Chen G, Li X, et al. Dynamic changes of D‐dimer and neutrophil‐lymphocyte count ratio as prognostic biomarkers in COVID‐19. Respir Res. 2020;21:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Doganci S, Ince ME, Ors N, et al. A new COVID‐19 prediction scoring model for in‐hospital mortality: experiences from turkey, single center retrospective cohort analysis. Eur Rev Med Pharmacol Sci. 2020;24:10247‐10257. [DOI] [PubMed] [Google Scholar]
- 85. Yang Q, Zhou Y, Wang X, et al. Effect of hypertension on outcomes of adult inpatients with COVID‐19 in Wuhan, China: a propensity score‐matching analysis. Respir Res. 2020;21:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Guo J, Zhou B, Zhu M, et al. CURB‐65 may serve as a useful prognostic marker in COVID‐19 patients within Wuhan, China: a retrospective cohort study. Epidemiol Infect. 2020;148. 10.1017/S0950268820002368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Abbattista M, Ciavarella A, Capecchi M, et al. Risk factors for mortality in hospitalized patients with COVID‐19: a study in Milan, Italy. Infect Dis (Auckl). 2021;53:226‐229. [DOI] [PubMed] [Google Scholar]
- 88. Weng Z, Chen Q, Li S, et al. ANDC: an early warning score to predict mortality risk for patients with coronavirus disease 2019. J Transl Med. 2020;18:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Abrishami A, Eslami V, Baharvand Z, et al. Epicardial adipose tissue, inflammatory biomarkers and COVID‐19: Is there a possible relationship?. International Immunopharmacology.2021;90: 107174. 10.1016/j.intimp.2020.107174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Pakos IS, Lo KB, Salacup G, et al. Characteristics of peripheral blood differential counts in hospitalized patients with COVID‐19. Eur J Haematol. 2020;105:773‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Güneysu F, Guner NG, Erdem AF, Durmus E, Durgun Y, Yurumez Y. Can covid‐19 mortality be predicted in the emergency room? J Coll Physicians Surg Pakistan. 2020;30:928‐932. [DOI] [PubMed] [Google Scholar]
- 92. Laguna‐goya R, Utrero‐rico A, Talayero P, et al. IL‐6–based mortality risk model for hospitalized patients with COVID‐19. Journal of Allergy and Clinical Immunology. 2020;146 :799–807.e9. 10.1016/j.jaci.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang R, He M, Yin W, et al. The prognostic nutritional index is associated with mortality of COVID‐19 patients in Wuhan, China. J Clin Lab Anal. 2020;34:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Zhang S, Guo M, Duan L, et al. Development and validation of a risk factor‐based system to predict short‐term survival in adult hospitalized patients with COVID‐19: a multicenter, retrospective, cohort study. Crit Care. 2020;24:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kunal S, Sharma S, Sharma S, Gautam D, Bhatia H, Mahla H, Sharma S, Bhandari S. Cardiovascular complications and its impact on outcomes in COVID‐19. Indian Heart Journal. 2020;72:593–598. 10.1016/j.ihj.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. de Jager CPC, Wever PC, Gemen EFA, et al. The neutrophil‐lymphocyte count ratio in patients with community‐acquired pneumonia. PLoS One. 2012;7:4‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cataudella E, Giraffa CM, Di Marca S, et al. Neutrophil‐to‐lymphocyte ratio: an emerging marker predicting prognosis in elderly adults with community‐acquired pneumonia. J Am Geriatr Soc. 2017;65:1796‐1801. [DOI] [PubMed] [Google Scholar]
- 98. Liu X, Zhang R, He G. Hematological findings in coronavirus disease 2019: indications of progression of disease. Ann Hematol. 2020;99:1421‐1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wang S, Fu L, Huang K, Han J, Zhang R, Fu Z. Neutrophil‐to‐lymphocyte ratio on admission is an independent risk factor for the severity and mortality in patients with coronavirus disease 2019. Journal of Infection. 2021;82:e16–e18. 10.1016/j.jinf.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chan AS, Rout A. Use of neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios in COVID‐19. J Clin Med Res. 2020;12:448‐453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Kong M, Zhang H, Cao X, Mao X, Lu Z. Higher level of neutrophil‐to‐lymphocyte is associated with severe COVID‐19. Epidemiol Infect. 2020;148:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fathi N, Rezaei N. Lymphopenia in COVID‐19: therapeutic opportunities. Cell Biol Int. 2020;44:1792‐1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Henry BM, Cheruiyot I, Vikse J, et al. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID‐19: a meta‐analysis. Acta Biomed. 2020;91:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wynants L, Van Calster B, Collins GS, et al. Prediction models for diagnosis and prognosis of covid‐19: systematic review and critical appraisal. BMJ. 2020;369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lagunas‐Rangel FA. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis. J Med Virol. 2020;92:1733‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Dobler CC. Poor quality research and clinical practice during COVID‐19. Breathe. 2020;16:1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Data available on request from the authors—The data that support the findings of this study are available from the corresponding author upon reasonable request.


