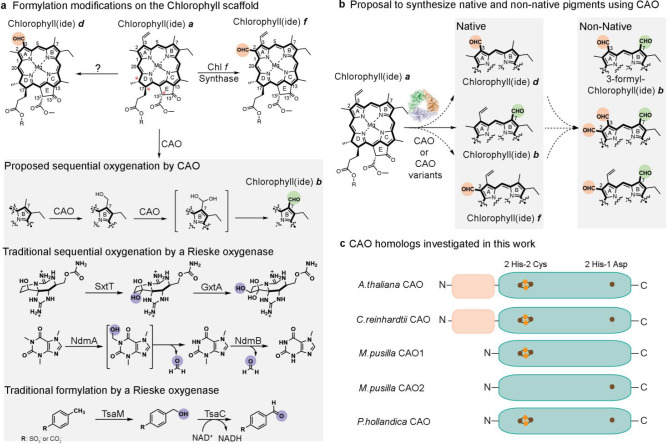
Figure 1.
Formyl groups are abundant modifications made on the chlorophyll (Chl) scaffold. (a) The pigments Chl b, d, and f each bear formyl groups on their macrocyclic chlorin scaffolds at various positions. These modifications contribute to their characteristic absorbance patterns. The modifications found in Chl d and f are formed using an as-yet unidentified enzyme and the photooxidoreductase Chl f synthase,1−3 respectively. Formation of the C7 formyl group in chlorophyll(ide) b is instead proposed to be installed via two sequential chlorophyllide a oxygenase (CAO)-catalyzed reactions that transform the C7-methyl group of chlorophyll(ide) a into the formyl group of chlorophyll(ide) b through C7-hydroxymethyl and C7-dihydroxymethyl intermediates.10 All three chiral centers in Chl a, b, and intermediates are labeled with a red asterisk and for the chemical structure, R = H or C20H39 for chlorophyllide and chlorophyll pigments, respectively. This proposal for CAO is unlike that for other transformations that proceed through more than one monooxygenation reaction and require two Rieske oxygenases to be completed.23−25 This proposal is also unlike that needed to convert a methyl group into a formyl group in the catabolism of 4-toluene sulfonate, which requires both a Rieske oxygenase and dehydrogenase.26 (b) CAO has potential to be used as a tool for formylating the Chl scaffold to produce custom-tuned native and non-native pigments. (c) The CAO homologues studied in this work have different domain architectures. All homologues of CAO are predicted to have a Rieske [2Fe-2S] cluster and a mononuclear nonheme iron site in their catalytic domains (blue). These metallocenters are coordinated by two His and two Cys ligands and a facial triad of residues, respectively. The Micromonas pusilla CAO homologue is found in two polypeptide chains and the Arabidopsis thaliana and Chlamydomonas reinhardtii homologues have N-terminal regulatory domains (peach).27 As PhCAO appears to contain the simplest architecture of all four homologues, it was the first CAO homologue characterized in this work.