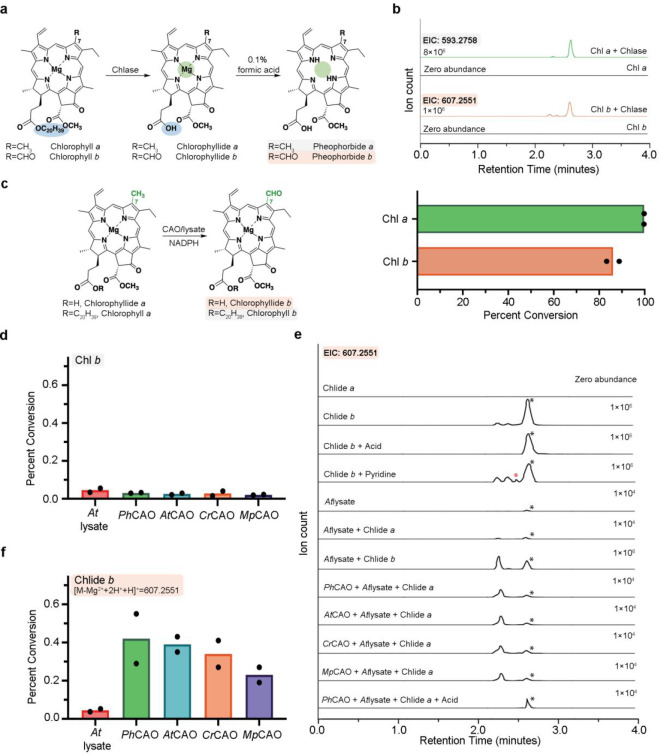
Figure 3.
CAO homologues transform Chlide a, not Chl a, into Chlide b in cell lysate. (a) Combination of a Chl pigment with recombinantly expressed and purified T. aestivum chlorophyllase (Chlase) permits formation of the desired Chlide a and Chlide b pigments. (b) Activity of chlorophyllase on Chl a and Chl b. Extracted ion chromatograms of the chlorophyllase activity assays product with Chl a (top trace) and Chl b (bottom trace). The LC-MS method designed and employed for pigment separation in this work relies on an acidic running solvent and causes loss of the central Mg2+ ion and the addition of two protons to the pigments under study. Therefore, the m/z = 593.2758 and m/z = 607.2551 represents the [M + H]+ of Chlide a and Chlide b minus Mg2+ plus 2H+, respectively (top panel). Chlorophyllase converts nearly 100% and 85% of Chl a and Chl b into their Chlide counterparts, respectively (bottom panel). (c) Proposed reaction scheme catalyzed by CAO in cell lysate. (d) None of the CAO homologues can transform Chl a into Chl b in A. thaliana cell lysate. (e) The extracted ion chromatograms for the CAO homologue reaction products when combined with a Chlide a substrate and A. thaliana cell lysate reveal that all four CAO homologues can convert Chlide a into Chlide b. Of note, the cell lysate, pyridine, and acid can each shift the diastereomer equilibrium of the Chlide b standard (traces 3–4 and 7). The black asterisk indicates the major peak of the standard, which is also observed in the assays and the red asterisk corresponds to the diastereomer peak observed in the enzymatic assays. (f) PhCAO shows the highest percent conversion among all the four homologues in the presence of A. thaliana cell lysate and a Chlide substrate. All data shown in the bar graphs was performed in duplicate and data are presented as mean values.