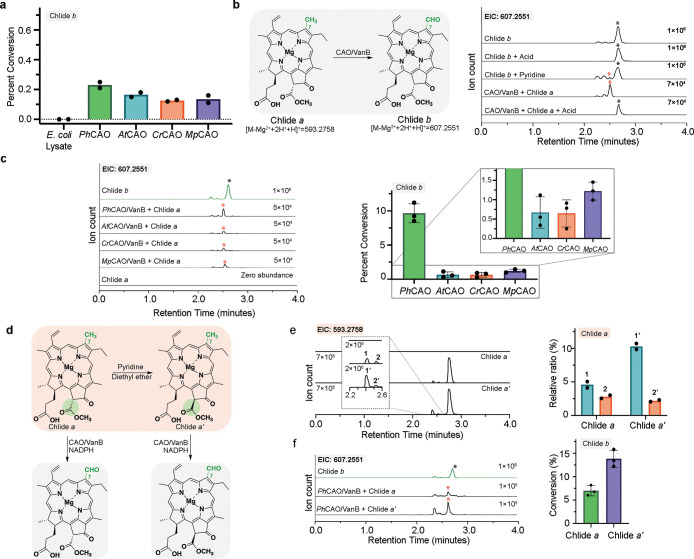
Figure 4.
CAO homologues transform chlorophyllide a into chlorophyllide b in the presence of the non-native reductase VanB. (a) All four CAO homologues show the ability to convert Chlide a into Chlide b in E. coli cell lysate, suggesting that a non-native reductase may work with CAO to facilitate the reaction. PhCAO shows the highest percent conversion among all four homologues. (b) Reaction scheme of the CAO/VanB catalyzed conversion of Chlide a into Chlide b (left panel). Treatment of the Chlide b standard with acid or pyridine shows new diastereomer peaks. Similarly, the reaction product from the CAO homologue reactions can be converted into the main diastereomer of the Chlide b standard under acidic conditions (right panel). (c) The extracted ion chromatograms for the product formed when the CAO homologues are combined with Chlide a, VanB, and NADPH. This data shows a diastereomer of Chlide b is formed in these reactions. Again, PhCAO shows the highest percent conversion among all four homologues. (d) Reaction scheme for converting Chlide a into Chlide a′ with pyridine. (e) An extracted ion chromatogram of the Chlide a peak suggests the Chlide a′ sample shows a different diastereomer distribution than Chlide a. (f) PhCAO shows a higher percent conversion on Chlide a′ than Chlide a. In panels c and f, reactions were performed in triplicate, and in panels a and e, they were performed in duplicate. In all bar graphs presented, data are shown as mean values.