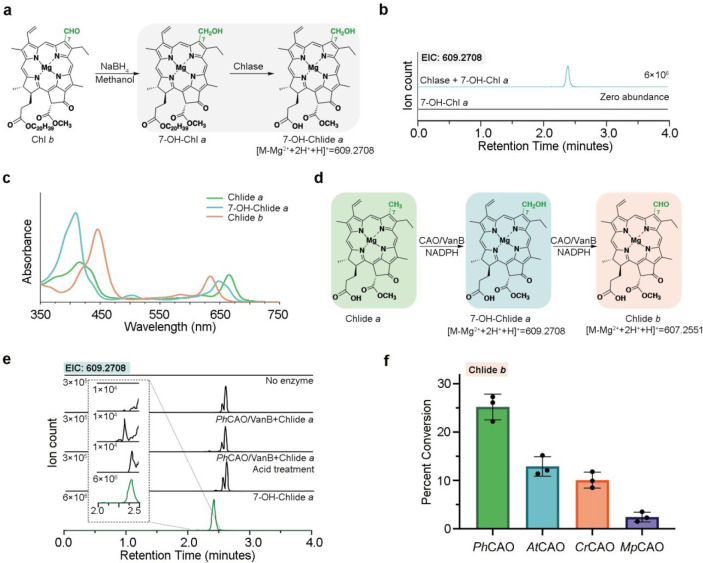
Figure 5.
7-OH-Chlide a can be transformed into Chlide b by combination of the CAO homologues with VanB and NADPH. (a) Reaction scheme for synthetic conversion of Chl b into 7-OH-Chlide a. (b) Extracted ion chromatograms of chlorophyllase catalyzed hydrolysis of 7-OH-Chl a. The m/z = 609.2708 represents the [M + H]+ of 7-OH-Chlide a minus Mg2+ plus 2H+ (see Figure 3a). (c) Absorbance spectra of the Chlide a, Chlide b, and 7-OH Chlide a that were enzymatically and synthetically produced in this work. (d) Reaction scheme of the oxygenation reactions that are catalyzed by CAO. (e) Extracted ion chromatograms for the PhCAO reaction products reveals the formation of a 7-OH-Chlide a intermediate. Acid treatment refers to a screening that was performed over a range of different pH values. This screening revealed that when the pH was adjusted to a value of 2, all Chlide b appeared as one peak. (f) All four CAO homologues show the ability to transform the intermediate (7-OH-Chlide a) into the final product (Chlide b). Again, PhCAO shows the highest percent conversion among all four homologues.