Figure 6.
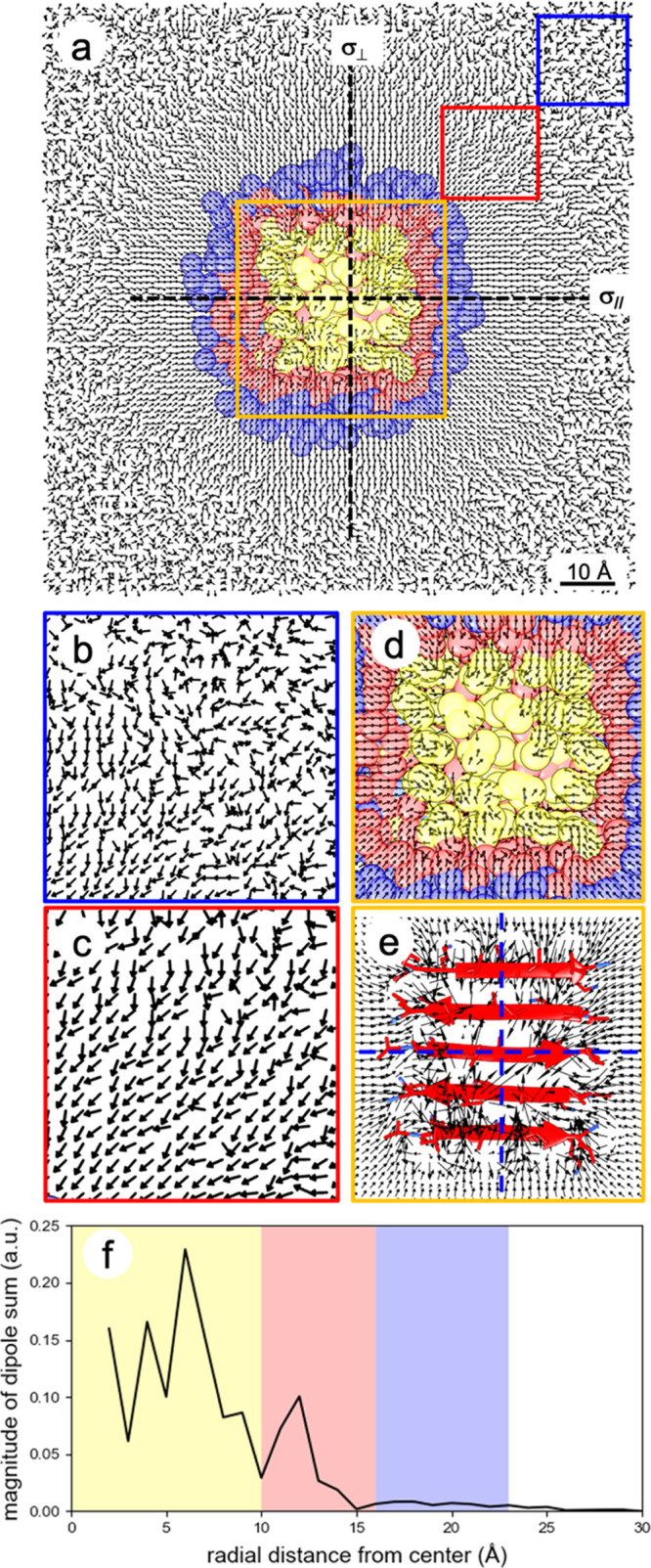
Symmetrical and asymmetrical orientations of water molecular dipole moments outside and inside the first hydration shell of the LK7β protein, respectively, at the vacuum–water interface. Water dipoles bisect the H–O–H angle and point toward the water oxygen. (a) Top view of water dipoles at the vacuum–water interface. The protein structure of LK7β (yellow) is the average conformation over the course of the MD trajectory. Red and blue regions, respectively, indicate the first and second hydration shells. Water dipoles point toward the protein due to the positive charges of the lysine residues. (b–d) Zoomed-in details of water dipoles in the blue, red, and orange boxes, respectively. (e) Zoomed-in details of water dipoles in the orange box, where the magnitudes of water dipoles are represented by the relative lengths of the arrows. For water dipoles outside the first hydration shell but still within ∼40 Å of the protein, two reflection planes perpendicular to the interface, namely, σ∥ aligning with and σ⊥ perpendicular to the β-strands, can be identified. For water dipoles inside the first hydration shell, no such reflection planes can be identified. (f) The magnitude of the vector sum of the water dipoles is plotted as a function of radial distance from the center of Figure 6a.
