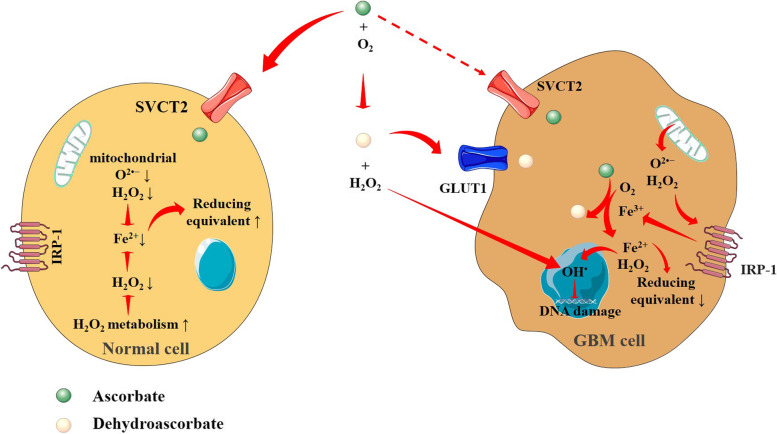
Fig. 4.
Differential response of ascorbate at a pharmacological dose on GBM cell and normal cell. The difference in H2O2 metabolism and iron metabolism is the signature of ascorbate selectivity toward cancer cells. Oxidation of ascorbate in the extracellular space produces H2O2 that enters the cell. In normal cells, ascorbate enters using SVCT2 and exhibits a high ability to metabolize H2O2, therefore iron metabolism is well maintained resulting in low cellular levels of labile iron (Fe2+). In GBM cells, dehydroascorbate enters using GLUT1; however, SVCT2 functionality is changed resulting in a change in the amount of ascorbate within cancer cells (dotted line) which hampers H2O2 metabolism. Subsequently, there is a build-up of H2O2 and disturbance in iron metabolism to enhance the levels of labile iron (Fe2+) within cancer cells. The intercellular conversion of ascorbate to dehydroascorbate aids in this process. The enhanced accumulation of cellular Fe2+ triggers free radical production, as well as decreased redox equivalent resulting in making GBM cells susceptible to vitamin C. Red arrows indicate downstream events and red lines indicate inhibition. ↑ indicates activation and ↓ indicates suppression. GLUT1, glucose transporter 1; H2O2, hydrogen peroxide; IRP1, iron regulatory protein 1; SVCT2, sodium-dependent vitamin C transporter 2