Abstract
In nonhuman animals, the phenomenon of rapid facial mimicry (RFM)—the automatic, involuntary, and rapid (<1 s) replication of others’ facial expressions—has been mainly investigated in the playful domain. In immature lowland gorillas Gorilla gorilla gorilla both play face (PF) and full PF (FPF) are rapidly mimicked between the players. This makes the species suitable to test hypotheses on the factors influencing RFM during play. The observations on 3 captive groups of lowland gorillas (N = 27) revealed that contrary to expectations, the closeness of social bond negatively influenced the occurrence of RFM but it did not affect either RFM latency or its overlapping index (OVERLAP). RFM was affected by the degree of symmetry of play fighting: the more balanced the session, the higher the occurrence of RFM. Players of the same sex class responded faster than players of different sex. These findings suggest that RFM may help synchronizing behaviors of playmates matching in size (same-sex) and promote symmetric playful interactions. “Laughing together” (measured by the RFM OVERLAP) lasted longer when the responder perfectly mirrored the partner expression (PF→PF; FPF→FPF). If PF and FPF convey information on the different play roughness degree, through “laughing together” the players could coordinate their actions and share positive moods and playful intensity. If the perfect congruency in the motor resonance, also known as social sensitivity, can foster a possible emotional dialogue between gorillas remains to be investigated.
Keywords: emotional resonance, facial overlapping, Gorilla gorilla gorilla, matching playmates, mirror response, play communication
The ability to read and appropriately interpret the signals emitted by conspecifics is an essential element for the success of sociality in humans and other highly social species (Freeberg et al. 2012; Demuru et al. 2015; Arnold and Winkielman 2020; Casetta et al. 2021; Nolfo et al. 2021). Among the bodily signals produced by primates, being more dynamic, nuanced, and rapid, facial expressions represent an efficient communicative channel employed in diverse social contexts, such as aggression, sex, and play (de Waal 1988; Palagi 2008; Cordoni and Palagi 2011a; Chen et al. 2018; Cordoni et al. 2018; Palagi et al. 2020a; Zannella et al. 2021). Having access to the face of the partner is therefore crucial to perceive and interpret their facial expressions in an appropriate way both at intra- and inter-specific level (Palagi and Mancini 2011; Prochazkova and Kret 2017; Annicchiarico et al. 2020; Maglieri et al. 2020). The strict linkage between facial signals emitted by the trigger and elicited in the receiver is a valuable measure to evaluate partners’ reciprocal attentional state (Palagi and Mancini 2011) and probably the perception and sharing of others’ emotional state (Nieuwburg et al. 2021). Facial reaction (or mimicry) allows individuals to detect contingencies in their social world and synchronize their activity thus limiting possible misunderstandings (Provine 1996, 2012; Palagi et al. 2019a, 2019b, 2020a, 2020,b; Nieuwburg et al. 2021).
Rapid facial mimicry (RFM) is a fast (<1 s) replication of others’ facial expressions (Palagi et al. 2015; Seibt et al. 2015; Prochazkova and Kret 2017; Clay et al. 2018; Minio-Paluello et al. 2020; Palagi et al. 2020a). The rapidity of the phenomenon suggests that RFM is an automatic and involuntary process (Dimberg and Thunberg 1998, 2012), possibly aimed at recognizing and synchronizing the emotional states of the interacting partners (Hess and Fischer 2013, 2014; Fischer and Hess 2017; Nieuwburg et al. 2021). Through the involuntary replication of others’ facial displays, the emotional state underpinning that behavior may be activated in the receiver (de Waal and Preston 2017; Olszanowski et al. 2019). In both humans and nonhuman animals, RFM is more frequent between subjects sharing close social bonds, such as in-group members, allies, friends, or kin (Homo sapiens: Bourgeois and Hess 2008; van Der Schalk et al. 2011; Hess and Fischer 2013; review on different nonhuman primate and other mammal species: Palagi and Scopa 2017; Canis lupus familiaris: Palagi et al. 2015; Suricata suricatta: Palagi et al. 2019a). Moreover, there is evidence that individuals sharing a higher level of familiarity or affiliation tend to respond with shorter latencies compared to strangers or unrelated individuals (Mancini et al. 2013a).
Since the linkage between RFM and social bond is readily detectable in social play (Davila-Ross et al. 2008; Mancini et al. 2013a, 2013b; Palagi et al. 2015, 2019a, 2019b; Taylor et al. 2019; Anderson and Kinnally 2021), this represents a useful behavior within which the complexity of facial communication in human and nonhuman animals can be explored (Palagi et al. 2016a).
Play mainly recruits motor patterns from other functional contexts (e.g., conspecific agonism, antipredator behavior, and mating), although they are temporally and structurally arranged in incomplete, fragmented, and disordered way (Burghardt 2005; Pellis and Pellis 2009; Palagi et al. 2016a). Such variability in the behavior patterns performed (Burghardt 2005) results in considerable unpredictability, requiring fine-tuned communication to be adequately managed (Bekoff and Allen 1998; Kraus et al. 2019;van Leeuwen et al. 2011). In this way, playful motivation is efficiently communicated between the partners, thus ensuring that the interaction remains symmetrical (i.e., balanced) and so reduces the risk of escalation to overt aggression (Palagi et al. 2015, 2019b). Hence, “mutual playful facial chattering” produces significant benefits to the subjects (Palagi and Mancini 2011) because it supports one of the activities that has a critical role in the ontogeny and maintenance of fitness-enhancing behaviors (Smaldino et al. 2019).
In nonhuman primates, the relaxed open-mouth display or play face (PF), a facial expression homologous to the visual component of human laughter (van Hooff 1972; Davila-Ross et al. 2009, 2010), is a visual signal largely used during playful interactions (van Hoof and Preuschoft 2003; Palagi 2007; Cordoni and Palagi 2011a, 2011b). Recently, some studies revealed that not only PFs are performed more frequently toward visually attentive receivers (Demuru et al. 2015; Aychet et al. 2021), but they are also rapidly mimicked by the receivers (Davila-Ross et al. 2008; Mancini et al. 2013a; Scopa and Palagi 2016; Taylor et al. 2019). Although what has been reported as examples of the PF can vary markedly between species and contexts (see Table 1, Pellis and Pellis 1996), and even within a species the same recognizable version of the PF can be highly graded (Parr et al. 2007), 2 variants of this facial expression can be readily distinguished and so used to assess congruent and incongruent signaling between partners. These are the PF, in which the mouth is open in a relaxed way with the only lower teeth visible, and the full PF (FPF) in which both the upper and lower teeth are visible (Palagi et al. 2007, 2019b; Waller and Cherry 2012).
Table 1.
Estimated parameters, SE, and results of the LRT of the GLMM (RFM presence/absence, binomial error distribution)
| Fixed effects | Estimate | SE | df | LRT | P |
|---|---|---|---|---|---|
| Intercept | 0.584 | 0.230 | a | a | a |
| Kinship (related)b,c | 0.468 | 0.284 | 1 | 2.717 | 0.099 |
| Age class combinationb,c (different age) | 0.076 | 0.227 | 1 | 0.107 | 0.744 |
| Sex class combinationb,c (different sex) | –0.223 | 0.212 | 1 | 1.166 | 0.280 |
| Social bonding | –1.436 | 0.648 | 1 | 4.830 | 0.028 |
| PAIabs | –1.253 | 0.373 | 1 | 11.441 | 0.001 |
n cases = 512; ndyads = 47. marginal R2 (theoretical) = 0.114; marginal R2 (Delta) = 0.091; conditional R2 (theoretical) = 0.117; conditional R2 (Delta) = 0.094. Variance for the random factor DYAD = 0.171 (± 0.413 SD); GROUP = 0.000. Control predictor duration (seconds) → estimate = 0.011 (± 0.003 SE); df = 1; LRT = 15.652; P = 0.001. The significant p-values are indicate in bold.
Not shown as not having a meaningful interpretation.
Estimate ± SE refers to the difference of the response between the reported level of this categorical predictor and the reference category of the same predictor.
These predictors were dummy coded, with the “Kinship (unrelated)”, Age class combination (same age)”, “Sex class combination (same sex)” being the reference categories.
In some primate species, both facial expressions have been observed. It has been proposed that the FPF includes morphological and functional elements of both the bared-teeth (a facial expression signaling appeasement) and PF displays (chimpanzees, Pan troglodytes, Preuschoft and van Hooff 1995; geladas, Theropithecus gelada, Palagi and Mancini 2011; Tonkean macaques, Macaca tonkeana, Scopa and Palagi 2016).
In lowland gorillas, immature animals are extremely playful (Forcina et al. 2019) and engage in the 2 variants of playful facial expressions (Waller and Cherry 2012; Palagi et al. 2019b). Similar to the other primate species, the FPF in gorillas seems to be a blend of the PF and the bared-teeth display (Parr et al. 2005). Moreover, studies on lowland gorillas have shown that the FPF was more frequently performed (mainly during rough play) and had a longer duration than the PF (Waller and Cherry 2012). Finally, a recent study on RFM during playful interactions showed that lowland gorillas are not only able to rapidly mimic others’ facial expressions, but also they do so by mirroring the exact facial variants (PF→PF; FPF→FPF; Palagi et al. 2019a). For all these reasons, the lowland gorilla is a good model species to test hypotheses on individual and social variables possibly influencing the RFM phenomenon during playful contacts.
Hypothesis 1: RFM as a means to share positive emotions.
If RFM is a socially modulated phenomenon being more pronounced between closely related individuals with the function to share playmates’ positive mood (Palagi et al. 2020b; Anderson and Kinnally 2021), we expect it to be more frequent between subjects showing a higher level of familiarity in terms of kinship and/or social bond (measured by grooming and body contact) (Prediction 1a). Moreover, we also expect that kinship and close social bonds shared by players significantly shorten the reaction time of the responder (Prediction 1b) and prolong “laughing together” events (Prediction 1c).
Hypothesis 2: RFM has the function to manage the asymmetry of the playful session.
If RFM is a valuable tool to manage playful asymmetry, we expect that the most balanced play sessions are characterized by a higher probability of RFM (Prediction 2).
Hypothesis 3: Facial mirroring affects the “laughing together” phenomenon.
The performance of distinct variants of PFs, as shown in lowland gorillas, is a necessary prerequisite for the mirror facial mimicry to occur (Palagi et al. 2019b; Taylor et al. 2019). However, no data are available in literature on the role of the mirroring (or matching) facial response on “laughing together”, defined as the amount of time animals overlap their facial expressions. If the mirroring of exact facial expressions (PF→PF; FPF→FPF), indicating high levels of social sensitivity, affects and synchronizes the engagement of the 2 players, we expect that the overlapping of the facial expressions emitted by the players (i.e., laughing together) during an RFM event is prolonged in case of their exact matching responses (Prediction 3).
Materials and Methods
The study colonies
The Beauval colonies
We observed 16 lowland gorillas belonging to a family and a bachelor group, respectively (see Supplementary Table S1). The 2 colonies were hosted at the ZooParc de Beauval (St Aignain Sur Cher, France) and occupied 2 similar enclosures composed of an indoor and outdoor facility of ∼200 m2 and 2.000 m2, respectively. The enclosures were comparable in terms of hiding (e.g., vegetation, rocks, and holes) and resting places (e.g., hammocks and platforms). The indoor facilities were furnished with trunks, lianas, and ropes. All the outdoor facilities were delimited by an artificial moat. The management schedule of the 2 groups was the same. Gorillas received abundant food (e.g., vegetables, seeds, grains, and branches with green leaves) 4 times per day approximately at the same hours each day. Twice a week the colonies received environmental enrichments such as sticks, rags, and small plastic tanks.
The Stüttgart colony
The colony was composed of 11 lowland gorillas (Supplementary Table S1) housed in the Wilhelma Zoological and Botanical Garden (Stüttgart, Germany). Although the enclosure comprised both an indoor and an outdoor facility, due to the severity of the weather conditions, the colony was exclusively observed in the indoor facility (∼600 m2 including 14 private, off-exhibit zones). The indoor facility was enriched with ropes, lianas, trunks, a small pond, and resting places such as platforms and hammocks. Animals received abundant food (e.g., vegetables, seeds, grains, and yogurt) 3 times a day approximately at the same hours each day. Once a day they also received environmental enrichments such as rags, paper boxes, small plastic tanks, and balls.
For all the observed groups water was available ad libitum and none of the individuals showed stereotypic or aberrant behaviors during the study period.
Based on Cordoni et al. (2018), we classified the gorillas under study according to the following age categories: infants (0–3.5 years), juveniles (3.5–6 years), adolescents (7–8 years), adult females (>8 years), blackback adult males (9–12 years), and silverback adult males (>12 years). In this study, infants and juveniles were considered immature individuals (Cordoni et al. 2018). The lowland gorillas of the 3 colonies did not differ in terms of age (Kruskall–Wallis test chi-square = 0.094; NBeauval_family = 11; NBeauval_bachelor = 5; NStüttgart = 11; P = 0.954).
Data collection
Observation schedule and video recording
Data were collected from October to December 2015 in the Beauval colonies (2 cameramen) and from January to March 2018 in the Stüttgart colony (2 cameramen). For all groups, the observations were carried out 6 days/week over a 6-h period covering both morning and afternoon and including feeding times. The 2 data collections were preceded by a training period of ∼35 h during which the observers became skilled in animal recognition, identification of the playful, grooming, and body contact events (the training was provided by E.P.). An animal was followed simultaneously by all the observers (Beauval: E.P., Serena Pressi, Maria Bobbio; Stuttgart: E.P., C.B.) and the data were later transcribed and compared. When a Cohen’s kappa values per each of the 3 events reached the 0.85 score (Kaufman and Rosenthal 2009) the training was interrupted for commencing the systematic video-data collection (digital videocameras employed: Panasonic HC-V180EG-K full-HD 50x, Sony HDR-PJ240, and full-HD Panasonic HC-V180 50x).
Play recording
In all the colonies under study, the all occurrences sampling method was employed in order to video-collect all the instances of play (Altmann 1974). A play session began when an individual directed any playful pattern toward a groupmate. If the partner ignored the invitation, this interaction was discarded from the analyses. A session ended when the players ceased to interact because moving away or being interrupted by a third individual. After an interruption of the bout of 10 s, the resumption of the interaction was considered as a new play session. The duration of each play session has been calculated in seconds. Since the forthcoming play in the great apes is easily predictable thanks to specific behavioral patterns/signals (Palagi et al. 2016), the observers could easily anticipate the interaction thus turning on the camera well before the beginning of each session. Due to the extreme low frequencies of adult play, we recorded it for the whole day. Play among immature individuals was recorded only for half of the observation period by switching between morning and afternoon on the following days to ensure a randomization of the observations across time. The total amount of video recording was 153 h for the Beauval groups and 426 for the Stuttgart colony. We collected and analyzed 647 playful events in the Beauval colonies and 401 in the Stüttgart colony. From this dataset, we extracted 208 playful sessions of the Beauval colonies and 304 of the Stüttgart colony. To be selected, a playful session had to include at least one event of PF/FPF detected by the potential receiver and the faces of both the trigger and the receiver had to be always visible to the observer in the video.
For each playful event, we recorded: 1) playmate identity (name, sex, and age, kinship); 2) each behavioral pattern in their sequential order (see Supplemental Table S2 for definitions); 3) playful facial expressions (PF and FPF—see Supplemental Table S2 for definitions) performed by partners as they occurred in chronological order; 4) the presence/absence of RFM (see below for the definition); and 5) exact duration (in csec (hundredths of a second)) of each behavioral pattern and playful expression. In particular, the duration of PF/FPF was calculated from the first video frame showing the separation between inferior and superior lips until the first frame showing the 2 lips closed again.
Affiliation recording
Via scan animal sampling (Altmann, 1974), we collected body contact and grooming interactions on the 3 colonies between group members at 10 min intervals (∼213 h of scan observation for the Stuttgart group and 106 for the Beauval groups). For determining the quality of the social bond between the subjects forming each playing dyad, we calculated the ratio between the number of grooming/body contact pattern observed and the total number of scans in which at least one of the players of the dyad was present.
In some studies on lowland gorillas, the measure of the quality of social bond includes also the levels of proximity between individuals (e.g., Stokes, 2004; Lemasson et al., 2018). Here, for comparison purposes, we applied the same methodology used in the RFM studies on other great ape species (bonobos, Palagi et al. 2020a, 2020b; chimpanzees, Palagi et al., 2019a, 2019b).
Operational definitions
Play Asymmetry Index calculation
In order to quantify the level of play asymmetry, for each session via a frame-by-frame analysis we calculated the Play Asymmetry Index (PAI; Cordoni et al. 2016, 2018) as follows: the number of offensive patterns directed by A toward B plus the number of defensive patterns performed by B toward A minus the number of offensive patterns directed by B toward A plus the number of defensive patterns performed by A toward B divided by the total number of playful patterns composing the session (i.e., offensive, defensive, and neutral patterns). The PAI ranges from – 1 (perfectly skewed toward player B) to +1 (perfectly skewed toward player A) with zero value indicating a complete symmetry of the session and absence of neutral patterns. For the classification of the playful patterns in offensive, defensive, and neutral see Supplementary Table S2.
RFM evaluation
RFM was considered to be present only if the latency between the detection and the response of the receiver was <1 s. To evaluate the presence/absence of RFM during playful interactions, we selected 2 different conditions: detection and no-detection. In the detection condition, we recorded the number of PF/FPF performed by the player when the face of the trigger (defined as the first player who emitted the facial stimulus) was directed toward the face of the playmate (direct visual contact condition, detection of the PF/FPF). In the no-detection condition, we recorded the number of PF/FPF emitted by the player when he/she was facing away from the face of the trigger who previously emitted the facial stimulus (without direct visual contact condition, no-detection of the PF/FPF). When the partner, who was initially looking away, turned his/her face toward the trigger that was still emitting a PF/FPF, this event was defined as detection condition, because the partner actually detected the stimulus. All the doubtful cases linked to lateral views were not included in the analysis.
It has been shown that primates are more likely to produce facial expressions when receiving visual attention from the interacting subject (Waller et al. 2015). Since during a session, there are many events in which the 2 players engage in mutual gazing, we compared the number of PF/FPF produced by the receiver when the trigger merely looked at the receiver’s face without producing any facial expression (response latency: 1 s starting from the first face-to-face engagement) vs. when the trigger looked at the receiver’s face and concurrently emitted a PF/FPF (response latency: 1 s starting from the perception of the PF/FPF).
Mirror mimicry evaluation
An event of RFM occurred when the trigger emitted a PF or FPF and the receiver responded independently either with a PF or FPF within a second (congruent response). Within the congruent responses (defining the RFM response), mirror rapid mimicry response was defined by the exact matching replication of the facial stimuli detected (PF→PF; FPF→FPF). A nonmirror rapid mimicry response was defined by a nonmatching replication of the facial stimuli detected (PF→FPF; FPF→PF).
RFM latency evaluation
The RFM latencies were measured frame-by-frame starting from the detection of the trigger PF/FPF stimulus and ending with the onset of the receiver’s PF/FPF (with 2-csec accuracy).
RFM overlapping index (OVERLAP) calculation
By applying the formula illustrated in Figure 1, we calculated the overlapping index (OVERLAP) of the PF/FPFs performed by the 2 players during each event of RFM. The calculation via this formula makes the OVERLAP independent from the time latency of the RFM event and allows balancing the overlapping value based on the duration of the PFs/FPFs.
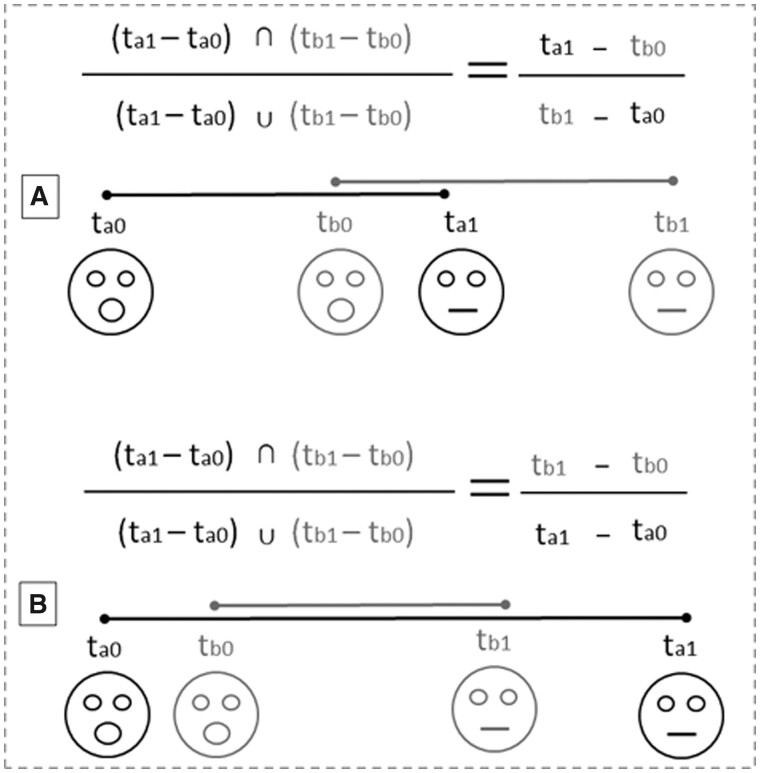
Figure 1.
Graphical formula illustrating the calculation of the OVERLAP between the play faces emitted by the subjects A and B. (tA1 = exact time of the end of PF/FPF by A; tB1 = exact time of the end of PF/FPF by B; tA0 = exact time of the beginning of PF/FPF by A; tB0 = exact time of the beginning of PF/FPF by B). The mathematical symbol ∩ indicates the intersection of 2 groups of data which corresponds to the time lag in common to the durations of the 2 facial expressions (tA1 − tB0). The mathematical symbol ∪ indicates the union of 2 groups of data corresponding to the time lag starting from the first frame showing the separation of the inferior from the superior lip of the subject A (tA0) to the first frame showing the 2 lips closed of the subject B (tB1). The calculation is applicable both when the PF/FPF of the trigger and responder overlap with each other and when player A’s PF/FPF ends later than player B’s PF/FPF ends (i.e., when the events occur in the following order: Player A’s PF/FPF onset → Player B’s PF/FPF onset → Player B’s PF/FPF offset → Player A’s PF/FPF offset).
Inter-observer agreement
The videotaped sequences collected were analyzed frame-by-frame and coded by using the program VideoLAN Client 2.2.1 and Jump-to-Time (VLC plug in). Before commencing systematic analysis of the videotaped sequences, the observers underwent a second round of training under the supervision of EP. During the video analysis, each combination of observers also including the trainer (E.P./S.P.; E.P./M.B.; S.P./M.B.; E.P./C.B.) scored the same 15 min of video. This procedure was repeated every 3 h of video analyzed to ensure consistent interobserver reliability for each behavioral item scored. This method allowed us to evaluate the interobserver reliability on ∼10.0% of the total amount of video recordings. The check was done for affiliation (grooming/body contact interactions), the playful offensive, defensive and neutral patterns, the PF and FPF and the detection/no-detection conditions (see Supplemental Table S2 for the definitions and explanations of the behaviors). The mean Cohen’s values of the behavioral patterns and the 2 conditions considered in the study were: Beauval: kdefensiveplay = 0.86, koffensiveplay = 0.85, kneutralplay = 0.87, kaffiliation = 0.93, kPF = 0.86, kFPF = 0.89, kdetectioN= 0.76; Stuttgardt: kdefensiveplay = 0.81, koffensiveplay = 0.83, kneutralplay = 0.90, kaffiliation = 0.95, kPF = 0.83, kFPF = 0.85, and kdetectioN = 0.75.
Statistical analyses
To compare the PF/FPF produced by the receiver when the trigger merely looked at the receiver’s face without producing any facial expression vs. when the trigger looked at the receiver’s face and concurrently emitted a PF/FPF, we carried out a paired permutation t-test at the dyadic level via randomization procedures with 10,000 shuffles (Manly, 1997) using the software Resampling Procedures 1.3 by David C. Howell (https://www.uvm.edu/~statdhtx/StatPages/Resampling/Resampling.html).
By using the lme4 package (Bates et al. 2015) in R (R Core Team, 2019; version 3.6.1), we ran a generalized linear mixed model (GLMM) and 2 linear mixed models (LMM). We ran a GLMM to test which variable affected the occurrence of RFM during each playful session (response variable: presence = 1, absence = 0 of RFM, binomial distribution). We only included in the analyses only the playful sessions where there was at least one PF/FPF perceived (N = 512). Each playful session was categorized by applying the following criteria: the category absence of RFM included the sessions with not-perceived PF/FPF (no-detection condition) or with at least one PF or FPF perceived (detection condition) but not mimicked by the potential receiver (N = 187) and the category presence of RFM included the sessions with at least one PF/FPF perceived (detection condition) and mimicked by the receiver within 1 s (N = 325). This procedure avoided the pseudo-replication of the data. The dyads included in the model were 49. The predictors included in the model were: kinship (related individuals = 1, coefficientkin = 0.5; unrelated individuals = 0, coefficientkin < 0.5), sex class combination (same sex318cases = 0; different sex194cases = 1), age class combination (same age class immature/immature340cases = 0; different age class immature/adult172cases = 1), social bonding (hourly frequency of grooming/contact sitting), and the absolute values of PAI. Dyads and groups (Beauval family group153cases = 0; Beauval bachelor group55cases = 1; Stuttgart group304cases = 2) were included as random factors.
A control predictor can be related to or affect the dependent variable, but it is not really of interest to the research question. Since the longer the session, the higher the probability to have RFM, in this first model the variable duration was entered as a control predictor.
Then, we ran an LMM which included the latency of the response in c-sec (Log-transformed variable, loglatency) as the response variable. The response was referred to the second subject forming each dyad. We determined the latency in rapid mimicry response by measuring frame-by-frame the time interval between the onset of the trigger stimulus and the onset of the receiver facial response (with 2-cs accuracy). The number of RFM events was 414 and the dyads involved were 29. The predictors included in the model were: kinship, sex class combination (same sex242cases = 0; different sex172cases = 1), age class combination (same age class immature/immature266cases = 0; different age class immature/adult148cases = 1), social bonding, the absolute values of PAI and the mirror/nonmirror response (PF→PF or FPF→FPF368cases; PF→FPF or FPF→PF46cases). Dyads and groups were included as random factors.
Finally, we ran a third model which included the OVERLAP of the rapid mimicry response in c-sec (Log-transformed variable, logOVERLAP) as the response variable. The response was referred to the second subject forming each dyad. The number of RFM events was 414 and the dyads involved were 29. The predictors included in the model were: kinship, sex class combination (same sex242cases = 0; different sex172cases = 1), age class combination (same age class immature/immature266cases = 0; different age class immature/adult148cases = 1), social bonding, the absolute values of PAI and the mirror/nonmirror response (PF→PF or FPF→FPF368cases; PF→FPF or FPF→PF46cases). Dyads and groups were included as random factors.
To exclude the occurrence of collinearity among predictors, we examined the variance inflation factors (vif package; Fox and Weisberg 2011). For the GLMM, we checked for the absence of residual overdispersion based on the ratio of residual deviance/degrees of freedom (df). Moreover, for the LMMs, we graphically checked for the normality of the residual distribution.
For GLMM analyses, we tested the significance of the full model (Forstmeier and Schielzeth 2011) by comparing it against a null model comprising the random factors and a control predictor, by using a likelihood ratio test (LRT) (Anova with argument test “Chisq”; Dobson 2002). For LMM analyses, the null model included only the random factors. Then, we calculated the p-values for the individual predictors based on LRTs between the full and the respective null model by using the R-function “drop1” (Barr et al. 2013).
Results
The mean duration of the playful sessions was 41.09 s ±1.92 standard error (SE) (total = 512 playful sessions). The mean number of PF/FPF performed per each session was 4.236 ± 0.150 SE. The mean number of RFM events per session was 2.021 ± 0.18 SE.
The 2-paired sample randomization procedure (permutation test) revealed that the number of PF/FPFs produced by the receiver when the trigger merely looked at the receiver’s face without producing any facial expression (mean 2.87 ± 0.38 SE) was significantly lower than when the trigger looked at the receiver’s face and concurrently emitted a PF/FPF (mean 5.54 ± 0.72 SE) (t = 5.291; ndyads = 61; P = 0.0001).
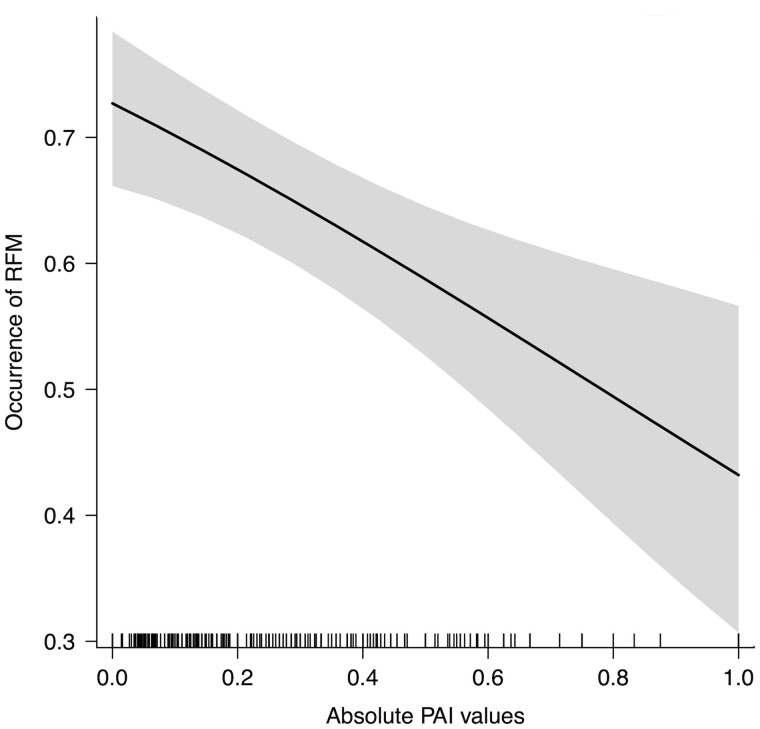
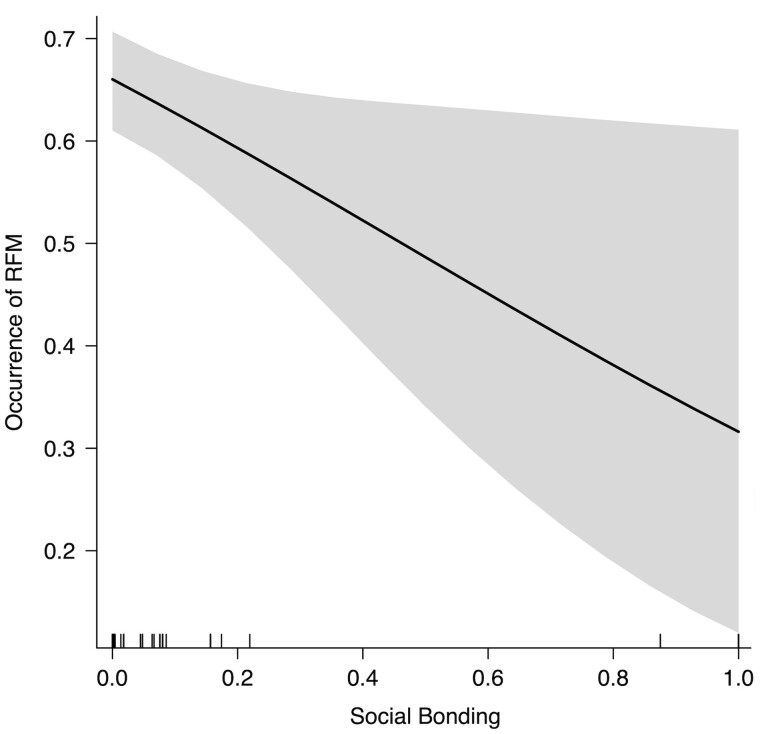
The full model (GLMM) built to analyze the variables possibly affecting the occurrence of RFM (binomial distribution: absence = 0, presence = 1) significantly differed from the control model comprising the random factors and the duration of the play session as control predictor (χ2 = 19.419, df= 5, P = 0.0016). The factors with a significant effect on the presence/absence of RFM were the absolute values of PAI (PAIabs) and social bonding (Table 1). There was a higher probability of the occurrence of RFM for the sessions characterized by lower absolute values of PAI (Figure 2, Prediction 2 supported) and when the subjects shared weaker social bonds (Figure 3, Prediction 1a not supported). No collinearity was found between the fixed factors (Min vif = 1.04; Max vif = 1.11). Moreover, no overdispersion was found (residual deviance = 627.851, df= 503, ratio = 1.248).
Figure 2.
Graph showing the relation between the absolute values of PAI and the predicted probability of RFM (occurrence of RFM). The line represents the linear regression between the 2 variables and the gray area represents the relative confidence interval.
Figure 3.
Graph showing the relation between social bonding (measured as grooming and contact sitting hourly frequency) and the predicted probability of RFM (occurrence of RFM). The line represents the linear regression between the 2 variables and the grey area represents the relative confidence interval.
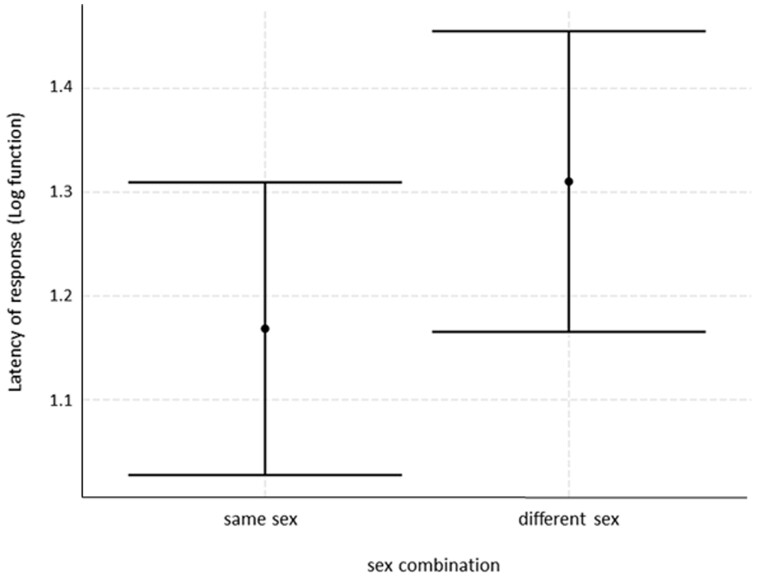
The second model (LMM) aimed at evaluating the variables possibly affecting the latency of the RFM by the responder (loglatency and normal distribution). The full model was statistically different from the null model (χ2 = 14.487, df= 6, P = 0.024). The fixed factor with a significant effect on the latency of the rapid mimicry response was sex class combination (Table 2). Specifically, the latency of response was lower when players matched in sex (male–male, female–female) (Figure 4). Neither social bond nor kinship affected the latency of RFM (Table 2, Prediction 1 b not supported). No collinearity was found between the fixed factors (Min vif = 1.01; Max vif = 1.21).
Table 2.
Estimated parameters, SE, df, values of the LRT, and probabilities of the linear mixed model (LOGlatency, continuous variable)
| Fixed effects | Estimate | SE | df | LRT | P |
|---|---|---|---|---|---|
| Intercept | 1.154 | 0.083 | a | a | a |
| Kinship (related)b, c | 0.015 | 0.062 | 1 | 0.102 | 0.749 |
| Age class combinationb, c (different age) | 0.093 | 0.057 | 1 | 3.008 | 0.083 |
| Sex class combinationb, c (different sex) | 0.142 | 0.057 | 1 | 6.584 | 0.010 |
| Social bonding | –0.002 | 0.002 | 1 | 0.594 | 0.441 |
| PAIabs | 0.129 | 0.114 | 1 | 1.212 | 0.271 |
| Mirror/no mirror responseb, c (no-mirror) | 0.128 | 0.079 | 1 | 2.583 | 0.108 |
n cases = 414; ndyads = 29. marginal R2 = 0.039; conditional R2 = 0.082. Variance for the random factor DYAD = 0.003 ± 0.056 SD; GROUP = 0.019 ± 0.139 SD).
Not shown as not having a meaningful interpretation.
Estimate ± SE refer to the difference of the response between the reported level of this categorical predictor and the reference category of the same predictor.
These predictors were dummy coded, with the “Kinship (unrelated)”, Age class combination (same age)”, Sex class combination (same sex)”, “Mirror/No mirror (mirror)” being the reference categories.
Figure 4.
Error bar showing the time latency of RFM (mean ± SE) as a function of the sex-class combination of the 2 players (same-sex/different sex).
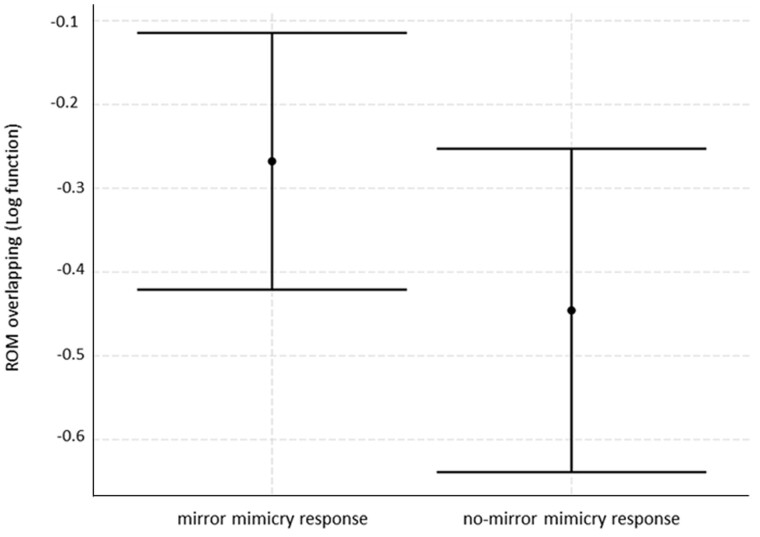
The third model (LMM) aimed at evaluating which variables possibly affected the OVERLAP (logOVERLAP, normal distribution) of the PF/FPFs performed by the 2 players during each event of facial mimicry. The full model statistically differed from the null model (χ2 = 13.93, df= 6, P = 0.030). The only fixed factor significantly affecting the OVERLAP was mirror/nonmirror response (PF→PF and FPF→FPF/PF→FPF and FPF→PF) (Table 3). Specifically, the OVERLAP was higher when the responder perfectly mirrored the facial expression of the trigger (PF→PF and FPF→FPF) (Figure 5, Prediction 3 supported). Neither social bond nor kinship affected the OVERLAP of facial expressions (Table 3, Prediction 1c not supported). No collinearity was found between the fixed factors in (Min vif = 1.01; Max vif = 1.20).
Table 3.
Estimated parameters, SE, df, values of the LRT, and probabilities of the linear mixed model (logOVERLAP, continuous variable)
| Fixed effects | Estimate | SE | df | LRT | P |
|---|---|---|---|---|---|
| Intercept | –0.285 | 0.085 | a | a | a |
| Kinship (related)b,c | –0.059 | 0.052 | 1 | 1.423 | 0.233 |
| Age class combinationb,c (different age) | –0.055 | 0.047 | 1 | 1.496 | 0.221 |
| Sex class combinationb,c (different sex) | –0.051 | 0.048 | 1 | 1.139 | 0.286 |
| Social bonding | 0.001 | 0.002 | 1 | 0.139 | 0.709 |
| PAIabs | 0.047 | 0.095 | 1 | 0.259 | 0.611 |
| Mirror/no mirror responseb,c (no-mirror) | –0.178 | 0.066 | 1 | 7.191 | 0.007 |
ncases = 414; ndyads = 29. Marginal R2 = 0.033; conditional R2 = 0.120. Variance for the random factor DYAD = 0.000 ± 0.000 SD; GROUP = 0.018 ± 0.135 SD)
Not shown as not having a meaningful interpretation.
Estimate ± SE refers to the difference of the response between the reported level of this categorical predictor and the reference category of the same predictor.
These predictors were dummy coded, with the “Kinship (unrelated)”, Age class combination (same age)”, Sex class combination (same sex)”, “Mirror/No mirror (mirror)” being the reference categories.
Figure 5.
Error bar showing the OVERLAP of the play faces (mean ± SE) emitted by the 2 players during RFM events as a function of the mirror (PF→PF/FPF→FPF) and the no-mirror mimicry response (PF→FPF/FPF→PF).
Discussion
Our findings on playful communication in lowland gorillas provide further insights into the mechanisms linked to the RFM phenomenon. Compared to other primate and nonprimate species (geladas, Mancini et al. 2013a; dogs, Palagi et al. 2015; meerkats, Palagi et al. 2019a), in which RFM seems to be promoted by social bonding, in lowland gorillas, RFM is socially modulated but in the opposite way. Indeed, in our study groups, close social bond negatively impacted on the probability of RFM to occur (Prediction 1a not supported). The closeness of social bonds between the players did not either shorten the time reaction of the responder (Prediction 1b not supported) or prolong the overlapping of the facial expressions (Prediction 1c not supported). In geladas, the peculiarity of facial mimicry between mother and offspring resides not only in their high levels of RFM but also in the reaction time (latency) of the mimicked events. Compared to the dyads formed by nonmother and infant geladas, mother–offspring RFM was characterized by the most rapid responses (Mancini et al. 2013a). In human infants, being imitated and imitating others can represent a nonverbal tool in communicating intentions to engage in future interactions and create social bridges and strong affiliation between play partners (Fawcett and Liszkowski 2012). Lowland gorillas do not frequently engage in affiliative activities such as grooming and contact interactions (Stokes et al., 2003; Stokes 2004; Masi et al. 2009). It is likely that, especially in the immature individuals, such low levels of social engagement are not sufficient to detect the different levels of familiarity between subjects thus making the relation between RFM and social bonding difficult to be highlighted. It is also possible that the occurrence of RFM between subjects characterized by low level of social affiliation can have a role in balancing their play fighting sessions. Contrary to the other African great apes, often engaging in frequent and prolonged body contact interactions (grooming sessions in chimpanzees, van Lawick-Goodall 1968; socio-sexual interactions in bonobos, de Waal 1995), social affiliation in lowland gorillas is probably expressed through different interactive behaviors such as spatial proximity and/or rapid contacts (e.g., touching, Watts 1995). Therefore, using these kinds of social interactions for measuring the quality of social bond between subjects could make the relation between RFM and social bonding easier to be detected. This could be worthy of further investigations.
According to our expectations, the most balanced playful sessions (measured by the PAI index) were characterized by the highest presence of RFM (Prediction 2 supported) (Table 1 and Figure 2). By having a role in balancing playful interactions, RFM could be more present when there is a higher risk of play escalation into aggression as it can occur when playmates match in their physical abilities and are poorly affiliated. Moreover, when the 2 players engage in a similar amount of offensive/defensive patterns the play session may become highly rewarding for both players (Kuczaj and Horback 2013). Probably, the linkage between matched playing style and emotional reward could lead to an increase in the expression of RFM.
The similarity of the players can also have a role in regulating the RFM phenomenon. We found that in the same-sex dyads individuals mimicked each other faster than in male–female dyads (Table 2 and Figure 3). We could argue that the similarity between the players can translate into the similarity of the play modality in terms of roughness, velocity, self-restraining, and facial expressions (Palagi et al. 2007). Although the variable age combination (same vs. different) failed to reach statistical significance (P = 0.083, see Table 2), this result could represent a valid starting point for further investigation on the relation between the similarity of ages of players and their motor synchronization. When matched players are involved and the play session is highly balanced, the RFM phenomenon could be more pronounced both in terms of occurrence and short latency. We have also to consider that different gorillas may have differing styles of play fighting and 2 same-sex individuals with divergent play styles may require greater communication to sustain the interaction.
During an RFM event, the overlapping of the playful expressions was longer when the responder perfectly mirrored the facial expression of the playmate (Prediction 3 supported) (Table 3 and Figure 4). In other words, when the 2 players shared the exact facial expression (PF→PF; FPF→FPF), they maintained their RFM interaction for longer (laughing together). It has been demonstrated that primates possess a dedicated and highly specialized cortical area to support face processing which in macaques is located in the region of the temporal lobe (Tsao et al. 2006). This region appears to be analogous and topographically homologous to the human fusiform face area (Schwarzlose et al. 2005). From a neurobiological perspective, we could hypothesize that the existence of such an area characterized by a high density of face-selective cells could be at the basis not only of a holistic face processing mechanism but also of a precise and highly selective facial expression recognition mechanism (Tsao et al. 2006). From an ethological viewpoint, several authors stated that the exposure of the upper teeth functions as an additional emphasis of nonaggression when intense play actions could be incorrectly interpreted (i.e., aggression) and observed that the upper teeth are exposed when play fighting becomes strongly vigorous (Loizos 1967; van Lawick-Goodall 1968; Palagi and Mancini 2011; Waller and Cherry 2012; Palagi et al. 2019b). Since the intensity of the playful facial expressions (PF vs. FPF) is a reliable indicator of the roughness degree of interaction, by mirroring the same facial expression and laughing together the 2 players could better coordinate their actions and regulate their reciprocal playful intensity.
Unfortunately, to our knowledge, no data on the OVERLAP between the RFM of partners are available in the same context in other species. We can only report a study on humans by Ichikawa and Makino (2007) who found that the facial expression of a sender had a longer duration when the receiver responded with a congruent facial expression rather than an incongruent one; although, it is worth noting that the 2 facial expressions analyzed in this study derive from different emotional domains (smiling and frowning).
In conclusion, the RFM phenomenon seems to be well represented in lowland gorillas and appears to have a role in the management of the playful sessions when the players are of the same sex, but not when they share a strong social bond. It is unclear if, as it occurs in other great apes, “laughing together” in gorillas can be a means for sharing positive mood and possibly managing the session. Hence, whether the perfect congruency in the motor resonance can foster a possible emotional dialogue during play remains to be investigated in this species.
Ethical notes
The research was purely observational and noninvasive and it complies with the current France, Germany, and Italian law and University regulations. Thus, no permit from the Bio-Ethical Committee of the University of Pisa (Italy) was needed.
Supplementary Material
Acknowledgments
Thanks are due to the ZooParc de Beauval (France) and the Wilhelma Zoo (Stuttgart, Germany) and the gorilla keepers of the two institutions for allowing and facilitating this study. In particular, we wish to thank, the head veterinarian and director of the research Beauval Nature, Baptiste Mulot (ZooParc de Beauval), and the curator of mammals Marianne Holtkötter (Wilhelma Zoo). Thanks are also due to Serena Pressi, Maria Bobbio, Giulia Annicchiarico, Marta Bertini for helping in the data collection.
Funding
Not applicable.
Supplementary Material
“Supplementary material can be found at https://academic.oup.com/cz”.
Conflicts of Interest Statement: The authors declare that they have no conflict of interest.
Ethics Approval
This is a non-invasive research compliant with the ASAB/ABS Guidelines for the Use of Animals in Research, the current Italian and German law, and University regulations. Thus, no permit from the Bio-Ethical Committee was needed.
Consent to Participate
Not applicable.
Consent for publication
Not applicable.
Availability of D ata and Material
Raw data are submitted as supplementary material.
Contributor Information
Chiara Bresciani, Natural History Museum, University of Pisa, Via Roma 79, Calci (Pisa) 56011, Italia.
Giada Cordoni, Natural History Museum, University of Pisa, Via Roma 79, Calci (Pisa) 56011, Italia.
Elisabetta Palagi, Natural History Museum, University of Pisa, Via Roma 79, Calci (Pisa) 56011, Italia; Department of Biology, Unit of Ethology, University of Pisa, Via A. Volta 6, Pisa 56126, Italia.
References
- Altmann J, 1974. Observational study of behavior: sampling methods. Behaviour 49:227–266. [DOI] [PubMed] [Google Scholar]
- Anderson JA, Kinnally EL, 2021. Behavioral mimicry predicts social favor in adolescent rhesus macaques Macaca mulatta. Primates 62:123–131. [DOI] [PubMed] [Google Scholar]
- Annicchiarico G, Bertini M, Cordoni G, Palagi E, 2020. Look at me while having sex! Eye-to-eye contact affects homosexual behaviour in bonobo females. Behaviour 157:949–970. [Google Scholar]
- Arnold AJ, Winkielman P, 2020. The mimicry among us: intra- and inter-personal mechanisms of spontaneous mimicry. J Nonverbal Behav 44:195–212. [Google Scholar]
- Aychet J, Blois-Heulin C, Palagi E, Lemasson A, 2021. Facial displays in red-capped mangabeys Cercocebus torquatus: repertoire, social context, and potential intentionality. J Comp Psychol 135:98–113. [DOI] [PubMed] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, Tily HJ, 2013. Random effects structure for confirmatory hypothesis testing: keep it maximal. J Mem Lang 68:255–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, Christensen RHB. et al., 2015. Package lme4. https://lme4.r-forge.r-project.org/ (Accessed 3 September 2021).
- Bekoff M, Allen C, 1998. Intentional communication and social play: how and why animals negotiate and agree to play. In: Byers JA, editor. Animal Play: Evolutionary, Comparative, and Ecological Perspectives. Cambridge: Cambridge University Press, 97–114. [Google Scholar]
- Bourgeois P, Hess U, 2008. The impact of social context on mimicry. Biol Psychol 77:343–352. [DOI] [PubMed] [Google Scholar]
- Burghardt GM, 2005. The Genesis of Animal Play: Testing the Limits. Cambridge: MIT Press. [Google Scholar]
- Casetta G, Nolfo PA, Palagi E, 2021. Yawn contagion promotes motor synchrony in wild lions Panthera leo. Anim Behav 174:149–159. [Google Scholar]
- Chen C, Crivelli C, Garrod OGB, Schyns PG, Fernández-Dols JM. et al., 2018. Distinct facial expressions represent pain and pleasure across cultures. Proc Natl Acad Sci USA 115:10013–10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay Z, Palagi E, de Waal FBM, 2018. Ethological approaches to empathy in primates. In: Meyza K, Knapska E, editors. Neuronal Correlates of Empathy: From Rodent to Man. Cambridge (MA): Elsevier Academic Press. pp. 53–66. [Google Scholar]
- Cordoni G, Palagi E, 2011a. Ontogenetic trajectories of chimpanzee social play: similarities with humans. PLoS ONE 6:e27344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordoni G, Palagi E, 2011b. Primate play laughing: a comparison between immature great apes and humans. J Biol Res 85:297–298 [Google Scholar]
- Cordoni G, Nicotra V, Palagi E, 2016. Unveiling the “secret” of play in dogs Canis lupus familiaris: asymmetry and signals. J Comp Psychol 130:278–287. [DOI] [PubMed] [Google Scholar]
- Cordoni G, Norscia I, Bobbio M, Palagi E, 2018. Differences in play can illuminate differences in affiliation: a comparative study on chimpanzees and gorillas. PLoS ONE 13:e0193096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila-Ross M, Menzler S, Zimmermann E, 2008. Rapid facial mimicry in orangutan play. Biol Lett 4:27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila-Ross M, Owren MJ, Zimmermann E, 2009. Reconstructing the evolution of laughter in great apes and humans. Curr Biol 19:1106–1111. [DOI] [PubMed] [Google Scholar]
- Davila-Ross M, Owren MJ, Zimmermann E, 2010. The evolution of laughter in great apes and humans. Communic Integrat Biol 3:191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal FBM, 1988. The communicative repertoire of captive bonobos Pan paniscus, compared to that of chimpanzees. Behaviour 106:183–251. [Google Scholar]
- de Waal FBM, 1995. Bonobo sex and society. Sci Am 272:82–88. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Preston SD, 2017. Mammalian empathy: behavioural manifestations and neural basis. Nat Rev Neurosci 18:498–509. [DOI] [PubMed] [Google Scholar]
- Demuru E, Ferrari PF, Palagi E, 2015. Emotionality and intentionality in bonobo playful communication. Anim Cogn 18:333–344. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, 1998. Rapid facial reactions to emotional facial expressions. Scand J Psychol 39:39–45. [DOI] [PubMed] [Google Scholar]
- Dimberg U, Thunberg M, 2012. Empathy, emotional contagion, and rapid facial reactions to angry and happy facial expressions. Psych J 1:118–127. [DOI] [PubMed] [Google Scholar]
- Dobson AJ, 2002. An Introduction to Generalized Linear Models. Baton Rouge (LA): Chapman and Hall. [Google Scholar]
- Fawcett C, Liszkowski U, 2012. Infants anticipate others' social preferences. Inf Child Devel 21:239–249. [Google Scholar]
- Fischer A, Hess U, 2017. Mimicking emotions. Curr Opin Psychol 17:151–155. [DOI] [PubMed] [Google Scholar]
- Forcina G, Vallet D, Le Gouar PJ. et al., 2019. From groups to communities in western lowland gorillas. Proc R Soc B 286:20182019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstmeier W, Schielzeth H, 2011. Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol 65:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J, Weisberg S, 2011. Multivariate Linear Models in R: An R Companion to Applied Regression. Thousand Oaks (CA): Sage. [Google Scholar]
- Freeberg TM, Dunbar RIM, Ord TJ, 2012. Social complexity as a proximate and ultimate factor in communicative complexity. Philos Trans R Soc Lond B Biol Sci 367:1785–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess U, Fischer A, 2013. Emotional mimicry as social regulation. Pers Soc Psychol Rev 17:142–157. [DOI] [PubMed] [Google Scholar]
- Hess U, Fischer A, 2014. Emotional mimicry: why and when we mimic emotions. Soc Personal Psychol Comp 8:45–57. [Google Scholar]
- Ichikawa H, Makino J, 2007. Function of congruent facial responses to smiling and frowning. Percept Mot Skills 105:838–851. [DOI] [PubMed] [Google Scholar]
- Kaufman AB, Rosenthal R, 2009. Can you believe my eyes? The importance of interobserver reliability statistics in observations of animal behaviour. Animal Behav 78:1487–1491. [Google Scholar]
- Kraus KL, Pellis VC, Pellis SM, 2019. Targets, tactics and cooperation in the play fighting of two genera of Old World Monkeys (Mandrillus and Papio): accounting for similarities and differences. Int J Comp Psychol 32:1–25. [Google Scholar]
- Kuczaj SA, Horback KM, 2013. Play and emotion. In: Watanabe S, Kuczaemotions S, editors. The Science of the Mind. Emotions of Animals and Humans: Comparative Perspectives. New York (NY): Springer, 87–111. [Google Scholar]
- Lemasson A, Pereira H, Levréro F, 2018. Social basis of vocal interactions in western lowland gorillas Gorilla g. gorilla. J Comp Psychol 132:141–151. [DOI] [PubMed] [Google Scholar]
- Loizos C, 1967. Play behaviour in higher primates: a review. In: Morris D, editor. Primate Ethology. Chicago (IL): Anchor Books, 226–282. [Google Scholar]
- Maglieri V, Bigozzi F, Riccobono MG, Palagi E, 2020. Levelling playing field: synchronization and rapid facial mimicry in dog-horse play. Behav Proc 174:doi.org/10.1016/j.beproc.2020.104104. [DOI] [PubMed] [Google Scholar]
- Mancini G, Ferrari PF, Palagi E, 2013a. Rapid facial mimicry in geladas. Sci Rep 3:1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G, Ferrari PF, Palagi E, 2013b. In play we trust: rapid facial mimicry predicts the duration of playful interactions in geladas. PLoS ONE 8:e66481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi S, Cipolletta C, Robbins MM, 2009. Western lowland gorillas Gorilla gorilla gorilla change their activity patterns in response to frugivory. Am J Primatol 71:91–100. [DOI] [PubMed] [Google Scholar]
- Minio-Paluello I, Porciello G, Gandolfo M, Boukarras S, Aglioti SM, 2020. The enfacement illusion boosts facial mimicry. Cortex 123:113–123. [DOI] [PubMed] [Google Scholar]
- Nieuwburg EGI, Ploeger A, Kret ME, 2021. Emotion recognition in nonhuman primates: how experimental research can contribute to a better understanding of underlying mechanisms. Neurosci Biobehav Rev 123:24–47. [DOI] [PubMed] [Google Scholar]
- Nolfo AP, Casetta G, Palagi E, 2021. Visual communication in social play of a hierarchical carnivore species: the case of wild spotted hyenas. Curr Zool zoab076. 10.1093/cz/zoab076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszanowski M, Wróbel M, Hess U, 2019. Mimicking and sharing emotions: a re-examination of the link between facial mimicry and emotional contagion. Cogn Emot 9:1–10. [DOI] [PubMed] [Google Scholar]
- Palagi E, 2007. Play at work: revisiting data focussing on chimpanzees Pan troglodytes. J Anthropol Sci 85:153–164. [Google Scholar]
- Palagi E, 2008. Sharing the motivation to play: the use of signals in adult bonobos. Anim Behav 75:887–896. [Google Scholar]
- Palagi E, Antonacci D, Cordoni G, 2007. Fine-tuning of social play in juvenile lowland gorillas Gorilla gorilla gorilla. Dev Psychobiol 49:433–445. [DOI] [PubMed] [Google Scholar]
- Palagi E, Bertini M, Annicchiarico G, Cordoni G, 2020a. Mirror replication of sexual facial expressions increases the probability of successful sexual contacts in bonobos. Sci Rep 10:18979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagi E, Burghardt GM, Smuts B, Cordoni G, Dall’Olio S. et al., 2016. Rough-and-tumble play as a window on animal communication. Biol Rev 91:311–327. [DOI] [PubMed] [Google Scholar]
- Palagi E, Celeghin A, Tamietto M, Winkielman P, Norscia I, 2020b. The neuroethology of spontaneous mimicry and emotional contagion in human and non-human animals. Neurosci Biobehav Rev 111:149–165. [DOI] [PubMed] [Google Scholar]
- Palagi E, Mancini G, 2011. Playing with the face: playful facial chattering and its modulation in a monkey species. J Comp Psychol 125:11–21. [DOI] [PubMed] [Google Scholar]
- Palagi E, Marchi E, Cavicchio P, Bandoli F, 2019a. Sharing playful mood: rapid facial mimicry in Suricata suricatta. Anim Cogn 22:719. [DOI] [PubMed] [Google Scholar]
- Palagi E, Nicotra V, Cordoni G, 2015. Rapid mimicry and emotional contagion in domestic dogs. R Soc Open Sci 2:150505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palagi E, Norscia I, Pressi S, Cordoni G, 2019b. Facial mimicry and play: a comparative study in chimpanzees and gorillas. Emotion 19:665–681. [DOI] [PubMed] [Google Scholar]
- Palagi E, Scopa C, 2017. Integrating Tinbergen’s inquiries: mimicry and play in humans and other social mammals. Learn Behav 45:378–389. [DOI] [PubMed] [Google Scholar]
- Parr LA, Cohen M, de Waal FBM, 2005. Influence of social context on the use of blended and graded facial displays in chimpanzees. Int J Primatol 26:73–103. [Google Scholar]
- Parr L, Waller BM, Vick SJ, Bard KA, 2007. Classifying chimpanzee facial expressions by muscle action. Emotion 7:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellis SM, Pellis VC, 1996. On knowing it’s only play: the role of play signals in play fighting. Aggression & Violent Behavior 1:249–268. [Google Scholar]
- Pellis SM, Pellis VC, 2009. The Playful Brain: Venturing to the Limits of Neuroscience. Oxford: Oneworld. [Google Scholar]
- Preuschoft S, van Hooff J, 1995. Homologizing primate facial displays: a critical review of methods. Folia Primatol 65:121–137. [DOI] [PubMed] [Google Scholar]
- Prochazkova E, Kret ME, 2017. Connecting minds and sharing emotions through mimicry: a neurocognitive model of emotional contagion. Neurosci Biobehav Rev 80:99–114. [DOI] [PubMed] [Google Scholar]
- Provine RR, 1996. Contagious yawning and laughter: significance for sensory feature detection, motor pattern generation, imitation, and the evolution of social behavior. In: Heyes CM, Galef BG Jr, editors. Social Learning in Animals: The Roots of Culture. San Diego (CA): Academic Press, 179–208. [Google Scholar]
- Provine RR, 2012. Curious Behavior: Yawning, Laughing, Hiccupping, and Beyond. Harvard: Harvard University Press. [Google Scholar]
- Schwarzlose RF, Baker CI, Kanwisher N, 2005. Separate face and body selectivity on the fusiform gyrus. J Neurosci 25:11055–11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopa C, Palagi E, 2016. Mimic me while playing! Social tolerance and rapid facial mimicry in macaques (Macaca tonkeana and Macaca fuscata). J Comp Psychol 130:153–161. [DOI] [PubMed] [Google Scholar]
- Seibt B, Mühlberger A, Likowski KUP, Weyers P, 2015. Facial mimicry in its social setting. Front Psychol 6:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaldino PE, Palagi E, Burghardt GM, Pellis SM, 2019. The evolution of two types of play. Behav Ecol 30:1388–1397. [Google Scholar]
- Stokes EJ, 2004. Within-group social relationships among females and adult males in wild western lowland gorillas Gorilla gorilla gorilla. Am J Primatol 64:233–246. [DOI] [PubMed] [Google Scholar]
- Stokes EJ, Parnell RJ, Olejniczak C, 2003. Female dispersal and reproductive success in wild western lowland gorillas Gorilla gorilla gorilla. Behav Ecol Sociobiol 54:329–339. [Google Scholar]
- Taylor D, Hartmann D, Dezecache G, Wong ST, Davila-Ross M, 2019. Facial complexity in sun bears: exact facial mimicry and social sensitivity. Sci Rep 9:4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao DY, Freiwald WA, Tootell RB, Livingstone MS, 2006. A cortical region consisting entirely of face-selective cells. Science 311:670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Der Schalk J, Fischer A, Doosje B, Wigboldus D, Hawk S. et al., 2011. Convergent and divergent responses to emotional displays of ingroup and outgroup. Emotion 11:286–298. [DOI] [PubMed] [Google Scholar]
- van Hooff J, Preuschoft S, 2003. Laughter and smiling: the intertwining of nature and culture. In: de Waal FBM, Tyack PL, editors. Animal Social Complexity: Intelligence, Culture, and Individualized Societies. Cambridge: Harvard University Press, 261–287. [Google Scholar]
- van Hooff J, 1972. A comparative approach to the phylogeny of laughter and smiling. In: Hinde RA, editor. Non-Verbal Communication. Cambridge: Cambridge University Press, 209–241. [Google Scholar]
- van Lawick-Goodall J, 1968. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim Behav Monog 1:161–311. [Google Scholar]
- van Leeuwen EJC, Zimmermann E, Davila-Ross M, 2011. Responding to inequities: gorillas try to maintain their competitive advantage during play fights. Biol Lett 7:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller BM, Cherry L, 2012. Facilitating play through communication: significance of teeth exposure in the gorilla play face. Am J Primatol 74:157–164. [DOI] [PubMed] [Google Scholar]
- Waller B, Caeiro CC, Davila-Ross M, 2015. Orangutans modify facial displays depending on recipient attention. PeerJ 3:e827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D, 1995. Post-conflict social events in wild mountain gorillas (Mammalia, Hominoidea). I. Social interactions between opponents. Ethology 100:139–157. [Google Scholar]
- Zannella A, Stanyon R, Maglieri V, Palagi E, 2021. Not all yawns tell the same story: the case of Tonkean macaques. Am J Primatol 83:e23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.