Abstract
Multimodal communication in animals is common, and is particularly well studied in signals that include both visual and auditory components. Multimodal signals that combine acoustic and olfactory components are less well known. Multimodal communication plays a crucial role in agonistic interactions in many mammals, but relatively little is known about this type of communication in nocturnal mammals. Here, we used male Great Himalayan leaf-nosed bats Hipposideros armiger to investigate multimodal signal function in acoustic and olfactory aggressive displays. We monitored the physiological responses (heart rate [HR]) when H. armiger was presented with 1 of 3 stimuli: territorial calls, forehead gland odors, and bimodal signals (calls + odors). Results showed that H. armiger rapidly increased their HR when exposed to any of the 3 stimuli. However, the duration of elevated HR and magnitude of change in HR increased significantly more when acoustic stimuli were presented alone compared with the presentation of olfactory stimuli alone. In contrast, the duration of elevated HR and magnitude of change in HR were significantly higher with bimodal stimuli than with olfactory stimuli alone, but no significant differences were found between the HR response to acoustic and bimodal stimuli. Our previous work showed that acoustic and chemical signals provided different types of information; here we describe experiments investigating the responses to those signals. These results suggest that olfactory and acoustic signals are non-redundant signal components, and that the acoustic component is the dominant modality in male H. armiger, at least as it related to HR. This study provides the first evidence that acoustic signals dominate over olfactory signals during agonistic interactions in a nocturnal mammal.
Keywords: acoustic, agonistic interactions, heart rate, Hipposideros armiger, multimodal communication, olfaction
Animals communicate using several sensory modalities simultaneously, a phenomenon known as multimodal communication (Partan and Marler 1999, 2005). Multimodal signals can be classified into redundant and non-redundant signals based on the classification framework of Partan and Marler (2005). The redundant signal hypothesis states that different components of multimodal signal convey similar information and elicit the same response of the receiver (Partan and Marler 1999, 2005; Bro-Jørgensen 2010). When components are combined, redundant signals might produce the same response (equivalence) or an increased response (enhancement; Partan 2013). In contrast, the multiple message hypothesis states different components of multimodal signal provide different information and produce different responses by the receiver (Partan and Marler 1999, 2005; Bro-Jørgensen 2010). When combined, each component of non-redundant signals can continue to have an effect (independence), 1 component can overshadow (dominance) or change (modulation) the effect of the other component, or their combination might provoke a new response (emergence; Partan and Marler 2005). It is important to examine receiver responses to isolated versus combined components because this approach can help us further classify multimodal signals (Partan and Marler 2005). The study of multimodal communication has attracted the attention of behavioral ecologists in the last 20 years because it is critical to fully understand animal communication (Partan and Marler 1999; Stevens 2013; Wierucka et al. 2018; Zhu et al. 2021). The accumulating evidence has been reported in both human and non-human primate communication via gestures and vocalizations because it is helpful to clarify human language origins (Fröhlich et al. 2019).
Territorial conflict presents an excellent multimodal context for investigating multimodal communication because it usually involves a number of sensory modalities including vision, acoustics, and olfaction (Briffa 2015). There can be strong selection on the assessment of fighting ability of opponents and on recognition of territory neighbors versus strangers (Arnott and Elwood 2009). The ability of a territory owner to recognize the status of its opponent and to assess the opponent’s fighting ability (i.e., mutual assessment) is beneficial because this facilitates an economic decision on whether to fight or flee (Arnott and Elwood 2009). Encoding information using multiple sensory channels provides increased signal information content thus reducing the likelihood of misidentification and increasing the accuracy of the assessment of a rivals’ fighting ability (Hebets and Papaj 2005; Frommen 2020). The multimodal signals in agonistic interactions have been studied in many non-mammals, such as the cichlid Neolamprologus pulcher (chemical and visual signals; Bayani et al. 2017), jumping spider Phidippus clarus (visual and vibratory signals; Elias et al. 2008), fiddler crab Uca mjoebergi (visual and vibrational signals Mowles et al. 2017), and 4 species of Sceloporus lizards (chemical and visual signals; Martins et al. 2018). The ability of multimodal signals to provide receivers with information about individual identity and fighting ability has also been well explored in a number of mammalian species (Vannoni and McElligott 2008; Faragó et al. 2014; Mercier et al. 2019; Sun et al. 2021). However, studies on multimodal communication during agonistic interactions in mammals have focused mainly on diurnal mammals that rely on visual and acoustic signals (e.g., Genty et al. 2014; Déaux et al. 2015), and no studies to date have tested nocturnal mammals that depend mostly on olfactory and acoustic signals. The addition of studies on signaling in nocturnal animals can help us fully understand the role of multimodal communication in conflict resolution.
The potential role of different components of multimodal signals has typically focused on changes in receiver behavior in response to isolated or combined components of the signal (Narins et al. 2003; Partan et al. 2009). While this approach is obviously important, it misses circumstances where a physiological response to a signal is stronger than an observable behavioral response, and this pattern may be particularly important under stressful conditions (e.g., Semin et al. 2019; Dal Bò et al. 2020; Groot et al. 2020). For example, heart rate (HR) could objectively quantify individual internal physiological changes in response to stimuli, especially if individuals do not display any visible behavioral responses to stimuli. HR has been confirmed to reflect internal physiological state (e.g., emotional arousal) in a number of contexts, including social behavior, animal cognition, and animal conflict (Wascher 2021). A positive relationship between HR and aggressiveness has been demonstrated in many mammals (Wascher 2021), including bats (Gadziola et al. 2012, 2016). Thus, HR is a suitable autonomic measure of a receiver’s response to multimodal signals generated during agonistic interactions.
The Great Himalayan leaf-nosed bat Hipposideros armiger is a nocturnal and highly gregarious mammal that usually roosts in caves, sharing day, and night roosts among hundreds of individuals (Cheng and Lee 2004). Our prior studies showed that male H. armiger defend their private roosting territories using multimodal displays incorporating territorial calls (acoustic signal; Sun et al. 2018) and forehead gland odors (olfactory signal; Zhang et al. 2020). Territorial calls of male H. armiger can contain information about emotional state, body mass, and individual identity (Sun et al. 2018, 2021), and forehead gland odors can encode information about threat and individual identity (Zhang et al. 2020).
The goal of this study was to categorize the multimodal aggressive display of male H. armiger using Partan and Marler’s (2005) classification framework. HR has been used as a proxy for response to signals during agonistic interactions (Wascher 2021). We thus used changes in HR of H. armiger to characterize the physiological response of bats both to multimodal territorial signals and to the isolated unimodal components of those signals. First, since our previous studies showed that territorial calls serve the function of territorial defense (Zhang et al. 2021) and olfactory signals serve the function of threat during the agonistic interactions (Zhang et al. 2020), we hypothesized that H. armiger would respond physiologically to acoustic, olfactory, and combined stimuli. We thus predicted that the instantaneous HR of H. armiger would increase when they were exposed to acoustic, olfactory, or combined stimuli. Second, we hypothesized that HR would respond to acoustic and olfactory signal components as they were redundant. We predicted that the combination of components would either produce 1) a similar response (equivalence) or 2) an increased response (enhancement) compared with either isolated component. The alternative hypothesis was that HR would respond to components as they were non-redundant. We predicted that 1 component would either 1) add to (independence), 2) override (dominance), 3) modulate (modulation), or 4) change (emergence) the information of the other.
Material and Methods
Collection and husbandry of bats
In April 2018, 8 adult males of H. armiger were collected with a mist net from the Shiyan cave in Chongyi, Jiangxi Province, China. Captured adults were maintained in a 6.5 m long × 5.5 m wide × 2.1 m high husbandry room. The room was maintained by Chun-Mian Zhang (CMZ) at a relative humidity around 60%, a temperature around 23°C, and a 12-h light/dark cycle, mimicking the natural environment inside the cave. All bats were given Zophobas morio larvae (super worms) ad libitum and freshwater enriched with vitamins and minerals. In order to identify individuals, we marked the bats with 4.2 mm metal rings (Porzana Ltd, East Sussex, UK) on their forearms. The bands do not affect the normal behavior of H. armiger (Sun et al. 2018). The 8 males were used for the subsequent sound recording, forehead gland secretion collection, electrocardiogram (ECG) monitoring, and stimulus presentation experiments. To reduce the potential effect of experience with familiar odors on the experiment, experimental bats were housed for at least 3 days in individual cages (0.5 m long × 0.5 m wide × 0.5 m high) in the husbandry room before the experiment started.
Sound recording and stimuli construction
Eight bats were used in our stimulus presentation experiments. Before running the experiments, territorial calls from each of these bats were recorded to use as the vocal stimuli in our experiment. This was done as follows: we placed the 8 bats into a large flight cage (4.4 m long × 1.5 m wide × 1.8 m high). Recording sessions were conducted from 20:00 to 08:00 the next day, which included the maximum activity period from 22:00 to 03:00. Vocalizations were recorded on 10 consecutive days. We used 2 infrared cameras (FDR-AX60; Sony Corp., Tokyo, Japan) to record their behaviors and used an Avisoft UltraSoundGate116H (Avisoft Bioacoustics, Glienicke, Germany) with an ultrasound microphone (CM16/CMPA, Avisoft Bioacoustics, Glienicke, Germany) to record their vocalizations. The sampling rate of the recording system was 250 kHz with 16-bit resolution. The distance between the microphone and the bat was 1.5 m. Camera 1 was placed at a distance of 30 cm from the ground to record the bats’ overall behavior. Camera 2 was placed at a distance of 120 cm from the ground to identify the caller. Our prior work (Sun et al. 2018) showed that male H. armiger emit bent-upward frequency modulated (bUFM) syllable calls, a common territorial call used in territory disputes, with the highest sound energy in the first harmonic during high-intensity aggression (Supplementary Figure S1a). Thus, we only recorded the bUFM syllable calls emitted by males during territorial disputes.
We created 8 playback files using Avisoft-SASLab Pro 5.2 (Supplementary Audio Files S1). Each playback file was created by randomly mixing 10 bUFM calls from 1 individual. A total of 562 syllables from 8 individuals (an average of 70 syllables per bat; range: 68–73; an average of 7 syllables per call; range: 4–12) were selected. The silent intervals between calls in the playback file were from 81 to 1,560 ms, mimicking the natural intervals of calls (Sun et al. 2018). Each playback file was 10 s long. The call rate was ∼1 call/s. The playback files were normalized so that the peak amplitude of the weakest call was ∼−30 dB. All playback files were high-pass filtered at 2 kHz to minimize the impact of background noise.
Forehead gland secretion collection and stimuli construction
The gland odors used in our experiments were collected as follows: We gently extruded a dark secretion from the forehead gland (Supplementary Figure S1B,C) by squeezing the area around the forehead gland, and transferred the secretion using pre-sterilized forceps into a 2 mL freezer tube vial with a PTFE-lined septum. We collected 2 samples from each of 8 individuals (16 samples in all) with a 15-day interval between the collection events. Two samples from each donor were needed because each experimental bat was presented with 1 unimodal (olfactory-only) and 1 multimodal (olfactory + acoustic) stimulus. All samples were collected between 19:00 and 19:30. To avoid cross-contamination between individuals, a pair of forceps was used for only 1 individual. After collection, we weighed the gland secretions to the nearest 0.001 g using an electronic balance (AR2140, Ohaus International Trading Co. Ltd, China). All samples were immediately stored at −80°C for subsequent experiments.
ECG monitoring
Individuals of some species of bats have been shown to produce aggressive vocalizations toward an acoustic stimulus, exhibiting wing flapping or boxing toward the acoustic stimulus, and hovering in front of the speaker (Fernandez et al. 2014). However, in our previous studies (Sun et al. 2018; Zhang et al. 2020), we rarely observed male H. armiger displaying these behavioral reactions to either acoustic or olfactory stimuli. We, therefore, used HR to evaluate the bats’ responses to aggressive signals. HR was monitored with an animal telemetry system (C19BTA, INDUS MouseMonitor Telemetry, Farmington Hills, MI, USA) using a sampling frequency of 512 Hz. Eight individuals underwent surgery to attach electrode spring tips that allowed an external ECG device (1.9 cc, 2.7 g, 22.0 mm × 12.0 mm × 7.5 mm) to connect with a communication module.
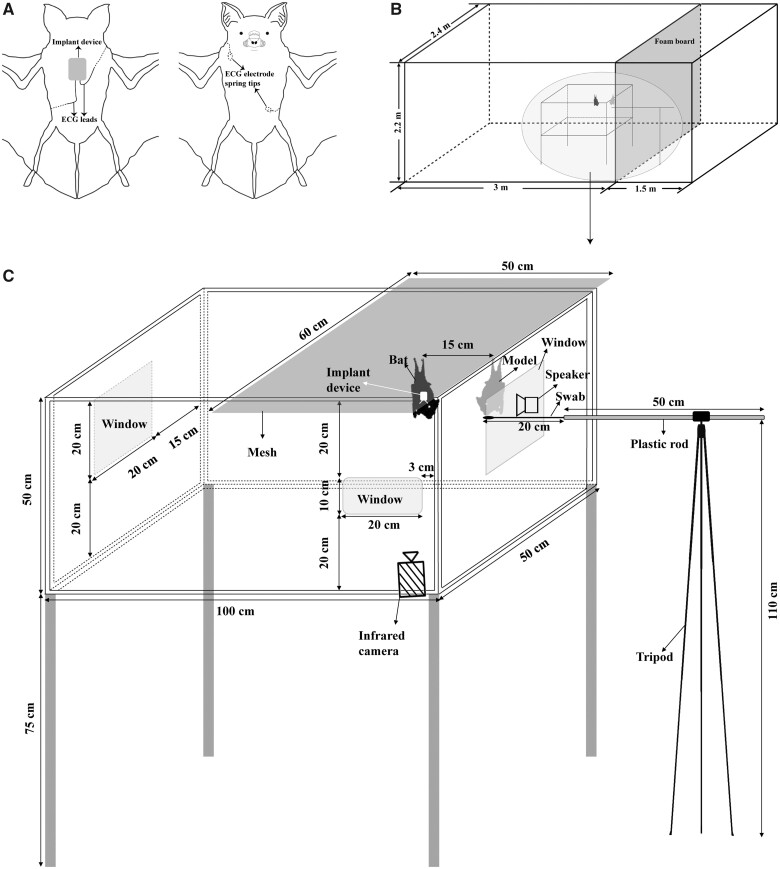
Prior to surgery, each bat received an intraperitoneal injection of sodium pentobarbital (1%, 40 mg/kg, i.p., Shanghai Haling Biological Technology Co. Ltd, China). After anesthesia, we placed the bats on a thermostat (S-100, Yuyan, China) at 30°C for surgery. Hair covering the dorsal and abdominal surface was removed with a shaver (327, LB, China). Four small incisions were made on the upper right of dorsal surface of the experimental bat, lower left of dorsal surface, upper left of abdominal surface, and lower right of abdominal surface. We wrapped the ECG device using plastic wrap so that it could be easily removed from the bat, and then we glued the ECG device onto the dorsal skin of bats using Deli 502 glue (Figure 1A). The ECG device consisted of positive and negative electrodes, and each electrode lead was covered with a plastic tube with a diameter of 2 mm. The purpose of this design was not only to prevent direct contact with air from reducing the electrical conductivity of the electrode lead, but also to make it easier to insert the electrodes in the subcutaneous tissues. The negative lead was inserted in the incision at the upper left of abdominal surface through to the incision at the upper right of dorsal surface of the experimental bat, and the positive lead was inserted in the incision at the lower right of abdominal surface through to the incision at the lower left of dorsal surface (Figure 1A). Subsequently, the electrode spring tips were attached to abdominal muscles using suture needles to prevent them from slipping in the subcutaneous tissues. The 4 incisions were closed with sutures after we ensured that the ECG was normal. Immediately following surgery, some antibiotic powder (Cefixime Dispersible Tablets, Kinhoo Pharmaceutical Co. Ltd, Zhuhai, China) was applied to the incisions to help wound healing. A square adhesive bandage was attached to the dorsal surface to prevent the bats from scratching the ECG device. The surgery lasted ∼20 min for each experimental bat. It took ∼30 min for the bats to recover from the anesthetic. After the bats woke up, they were housed in the individual cages (0.5 m long × 0.5 m wide × 0.5 m high) for a 1-week recovery period before the experiment. Bat movement was limited in these cages to allow for wound healing. They were then transferred to a larger cage where they were able to move around and feed freely.
Figure 1.
Experimental design. (A) Schematic diagram of ECG device implanted in the H. armiger on the back (left) and abdomen (right). The grey rectangle indicates ECG device. The device is above the skin. The 2 wires indicate positive and negative poles, respectively. Solid lines represent that the wires are above the skin, and dotted lines represent that the wires are under the skin. The 2 circles indicate the locations of the 2 electrode spring tips, and the 2 electrode spring tips are buried under the subcutis of the abdomen. (B) The overall view of the experimental setup for playback of different stimuli. The gray rectangle indicates a foam board. The area circled in gray is the experimental setup used for playback. (C) The partial enlargement of the experimental setup for playback of different stimuli. The gray bat indicates the taxidermic model. The black bat indicates the live bat, which roosts on the mesh. A speaker and a swab were placed next to the taxidermic model at the same horizontal level. An infrared camera was used to record the behavior of bats from the front window.
Stimulus presentation experiments
We conducted 3 types of stimulus presentation: acoustic, olfactory, and combined (multimodal). The experiments were carried out in a box made of plexiglass with no lid (1.0 m long × 0.5 m wide × 0.5 m high; Figure 1C) in a 4.5 m long × 2.4 m wide × 2.2 m high room (Figure 1B). We placed a taxidermic model inside the window where the stimuli were presented to simulate a territorial bat. We made a taxidermic model from a dead bat that was captured from the roosting cave. We placed a speaker (Ultrasonic Dynamic Speaker, Avisoft Bioacoustics, Glienicke, Germany) and a swab with gland secretions, which were at the same horizontal level and 1.1 m above the ground, outside the stimulus window (Figure 1C). An infrared camera from the front window was used to monitor whether the bats calmed down (i.e., remained motionless or started self-grooming) before recording the baseline HR. Before the trial, we randomly selected a male and hung it at a distance of 15 cm from the taxidermic model to acclimate it to the box. After the bat remained motionless for ∼5 min, we first recorded its HR for 5 min in resting state. Then, we recorded the HR for 5 min during a stimulus presentation. All bats were exposed to 1 stimulus each day. Each bat was tested every 1 or 2 days. Each bat was used only once with each stimulus. Experiments were conducted during the bats’ usual activity period (19:00–21:00). All stimuli presented to a single bat came from the same donor, and different bats were tested with stimuli from different donors.
When we broadcast acoustic stimuli, the amplitude of bUFM calls was ∼60 dB SPL at a distance of 15 cm from the bat. We calibrated the acoustic stimuli at a sound level of around 60 dB SPL at 1 m from the speaker by comparing the root mean square to a 15 kHz reference tone. We tested individual responses to olfactory stimuli using a swab fastened to a 50 cm long and 2 cm diameter stick. The stick was attached to a telescopic rod to allow the position of the swab to be adjusted with a rotating button on a tripod attached to the telescopic rod (Figure 1C). After we recorded HR for 5 min in the resting bat, we placed the 5 mg forehead gland secretions on the swab. Subsequently, the swab was moved toward the bat by rotating the button on the tripod. This process took ∼5 s and was not included in the statistical analyses. When the distance between the swab and the bat was 15 cm, we stopped rotating the button and started recording the HR of the bat. The duration of stimulus presentation was 5 min. To estimate individual responses to the multimodal stimulus, 1 experimenter made the gland secretion swab gently approach the bat. When the distance between the swab and the bat was 15 cm, another experimenter simultaneously broadcast bUFM calls and recorded the HR of the bat. The experimenters were quiet during all experiments.
ECG analysis
Instantaneous HR was calculated using the reciprocal of 2 successive R-wave intervals (R–R) in beats per minute (bpm). To reduce the impact of background “noise” on the ECG recordings, all voltages ˂0.35 mV were filtered from the data set. There were also outliers in the data set that were removed before instantaneous HR was calculated. We defined outliers as data points more than 2 standard deviations (SDs) from the mean or >1,200 bpm which was greater than the physiological maximum (Kurta and Baker 1990; Neuweiler 2000). After the outliers were removed, we used “NaN” to fill in the missing values. We combined all the data points of each 10-min experiment (5 min baseline plus 5 min stimulus presentation) into an Excel file, and the Excel files were imported into MATLAB R2019a (MathWorks, Natick, MA, USA). A shape-preserving segmented cubic interpolation was used to fill in the missing values. Instantaneous HR was boxed at 1 s, and a 10-s sliding window with 1-s increments was used for smoothing the data.
Baseline HR was computed as the mean instantaneous HR for the first 5 min of the experiments. The duration of elevated HR was computed as the duration of time that the instantaneous HR was greater than the mean baseline HR plus 2 SDs. The magnitude of change in HR was defined as the maximum of the instantaneous HR minus the mean baseline HR.
Statistical analysis
All variables in this study were normally distributed (Shapiro–Wilk tests; P > 0.05). One-way analysis of variance (ANOVA) was performed to compare the differences in the baseline HR, duration of elevated HR, and the magnitude of change in HR under different playback stimuli. If there were significant differences, Bonferroni multiple-comparison tests were used to test the differences in baseline HR, duration, and magnitude of elevated HR between any 2 types of stimuli. Statistical analyses were performed in SPSS version 22.0 (IBM Corp., Armonk, NY, USA). The significance level was set at P < 0.05. All data are presented as mean ± SD.
Results
Change in HR under different presentation stimuli
The average baseline HR of H. armiger under acoustic, olfactory, and multimodal stimuli was 359 ± 45, 351 ± 39, and 375 ± 51 bpm (Figure 2), respectively. There were no significant differences in the baseline HR between the 3 playback stimuli (ANOVA: F2,5 = 0.610, P = 0.553).
Figure 2.

Changes in instantaneous HR during three 10-min playback trials from 8 bats. Changes in instantaneous HR when H. armiger were at 5-min resting state (0–300 s) and 5-min acoustic stimuli (300–600 s; blue line), 5-min resting state (0–300 s) and 5-min olfactory stimuli (300–600 s; red line), and 5-min resting state (0–300 s) and 5-min multimodal stimuli (300–600 s; green line). The gray area represents 95% confidence interval.
There was a rapid increase in instantaneous HR when we presented either the acoustic, olfactory, or multimodal stimuli (Figure 2). These results suggest that bats could respond to different stimuli. The elevated HR was sustained for an average of 157 s and gradually returned to baseline after stimulus presentation for an average of 143 s (Figure 2).
Differences in HR under different playback stimuli
There were significant differences in the duration of elevated HR in response to the 3 stimulus presentations (ANOVA: F2,5 = 10.64, P= 0.001; Figure 3A). Bonferroni multiple-comparison tests indicated that these differences were significant between the acoustic (173 ± 55 s; Figure 3A) and olfactory (90 ± 55 s; P = 0.016; Figure 3A) stimuli, and between multimodal (210 ± 49 s; Figure 3A) and olfactory stimuli. However, there were no significant differences between the multimodal and acoustic stimuli (P = 0.532; Figure 3A).
Figure 3.
Comparison of the duration of elevated HR (A) and magnitude of change in HR (B) under 3 different stimuli. Blue solid points indicate only playback acoustic stimuli; red solid points indicate only playback olfactory stimuli; green solid points indicate playback acoustic combined with olfactory stimuli; lines indicate mean values of elevated HR (A) and magnitude of change in HR in each treatment (B). Different letters above values indicate significant differences under 3 different stimuli.
We also found that there were significant differences in the magnitude of change in HR among the 3 presentation stimuli (ANOVA: F2,5 = 11.388, P < 0.001; Figure 3B). Bonferroni multiple-comparison tests indicated that these differences were significant between the acoustic (186 ± 44 bpm; Figure 3B) and olfactory (85 ± 50 bpm; P = 0.001; Figure 3B) stimuli, and between the multimodal (182 ± 49 bpm; P = 0.002; Figure 3B) and olfactory stimuli. However, there were no significant differences in the magnitude of change in HR between multimodal and acoustic stimuli (P = 0.99; Figure 3B).
Discussion
In this study, we found that the instantaneous HR increased rapidly when H. armiger was exposed to acoustic, olfactory, or multimodal stimuli, supporting our first hypothesis that H. armiger showed a physiological response to signals typically emitted during territorial encounters. Moreover, when H. armiger was exposed to acoustic stimuli alone, the duration of elevated HR was significantly longer than bats exposed to olfactory stimuli alone. No significant differences were found in the duration of elevated HR and the magnitude of change in HR between multimodal and acoustic stimuli, while significant differences were found between multimodal and olfactory stimuli. These results are consistent with our previous findings that acoustic and chemical signals encoded different types of information (Sun et al. 2018, 2021; Zhang et al. 2020), supporting the second prediction of the third hypothesis (see the “Introduction” section) that olfactory and acoustic components are non-redundant, and that the acoustic component may be a dominant modality to odor during the territorial conflicts of H. armiger, at least as these relate to autonomic responses to these signals.
The rapid increases in instantaneous HR of male H. armiger were detected at the onset of acoustic, olfactory signals, and multimodal signals (Figure 2), which are known to be associated with agonistic interactions (Gadziola et al. 2016; Wascher 2021). HR is a key physiological trait and is significantly related to aggressiveness because it reflects emotional arousal levels of animals (Wascher 2021). Social stressors can trigger physiological responses in animals including changes in HR (Takahashi et al. 2018). More specifically, social stressors can activate the sympathetic nervous system to release catecholamines that can trigger an increase in HR (Stoddard et al. 1986; Sapolsky 1992). This increase in HR after social exposure to stressors has been explored in a number of mammalian species (Stöhr 1986; Jong et al. 2000; Polheber and Matchock 2014), including humans (Hamidovic et al. 2020). Intraspecific confrontation is one of the most common sources of social stressors among animals (Martinez et al. 1998; Konoshenko et al. 2013). High levels of aggressiveness have been shown to correlate with a higher HR in a number of vertebrates such as big brown bat Eptesicus fuscus (Gadziola et al. 2012, 2016), greylag geese Anser anser (Wascher et al. 2009), and domestic pigs (Marchant et al. 1995). Our results indicated that acoustic and olfactory signals in male H. armiger during agonistic interactions may represent social stressors to receivers. Increases in HR, modulated by higher emotional arousal during agonistic interactions, may also be indicative of higher levels of aggressiveness in territorial conflicts in male H. armiger.
We found that the change in HR in response to an acoustic signal was the same as the response to a multimodal (acoustic + olfactory) signal (Figure 3). In contrast, the HR response to an olfactory signal was significantly weaker than the response to the multimodal (acoustic + olfactory) signal. Thus, according to the classification of Partan and Marler (2005), acoustic and olfactory signal components in H. armiger appear to be non-redundant. Non-redundancy in multimodal signals is very common (Hebets 2008; Déaux et al. 2015; Chabrolles et al. 2017). Our previous work showed that male H. armiger bUFM calls can encode information about emotional state, body mass, and individual identity (Sun et al. 2018, 2021), and forehead gland secretions can encode information about threat and individual identity (Zhang et al. 2020). Together with these previous results, our study suggested that acoustic and olfactory components are non-redundant.
What is the benefit to male H. armiger of displaying non-redundant multimodal signals during agonistic interactions? One possible benefit indicated in the multiple messages hypothesis is that each component of the multimodal signal provides information about different aspects of a sender’s quality, thus allowing receivers to more rapidly and comprehensively evaluate senders’ quality (Møller and Pomiankowski 1993; Johnstone 1996). Hipposiderosarmiger has a polygynous mating system with 1 territorial male and several females (Yang 2011). Therefore, selection on territory holders to effectively communicate their individual identity and resource holding potential (RHP; i.e., factors such as body size, social rank, and aggressive motivation) is especially important in male H. armiger. Because males defend access to females/territory against other males, the ability of males to recognize the identity of neighboring males and assess the RHP of potential competitors based on acoustic and olfactory signals could facilitate neighbor-stranger discrimination (sensu, the “dear-enemy effect” that reduced aggression between established territorial neighbors relative to strangers; Ydenberg et al. 1988; Temeles 1994), and minimize costly aggressive interactions. These results suggested that acoustic and olfactory components of male H. armiger multimodal signals may provide different types of information of an opponent’s quality that in turn may facilitate individual recognition and assessment of fighting ability, and thereby facilitate conflict resolution without actual fighting.
Further, we found that the acoustic sensory modality may dominate over the olfactory modality in conflict resolution of H. armiger. One possibility is that the production of acoustic signals may be easier and quicker than that of olfactory signals because the formation and release of many olfactory signals tend to require an accumulation of material (Wyatt 2014). Moreover, the fact that glands may only exude a minimal level of secretion or even a complete lack of secretion during some periods (Chun-Mian Zhang, personal observation; Zhang et al. 2020) may weaken the value of olfactory signals in individual recognition and assessment. Thus, olfactory signals may be inappropriate as a dominant sensory modality for communication in male H. armiger. Together, these results indicate that acoustic signals should dominate olfactory signals during agonistic interactions in male H. armiger.
Although olfactory signals alone generated a weaker physiological response than either acoustic signals alone or the combination of acoustic and olfactory signals, olfactory signals still can play a role in agonistic interactions of male H. armiger. Multimodal communication of many mammals involves an olfactory sensory component, indicating that olfactory signals serve an important role in social interactions (Wierucka et al. 2018). Indeed, olfactory signals play an essential role in agonistic interactions in many mammals, including territorial defense (Jordan et al. 2007) and the modulation of aggressive behavior (Stritih and Kosi 2017). In bats, 15 species have been reported to have an odor-producing gland or structure (Muñoz-Romo et al. 2021). Most of the glands and odor-dispersing structures are sexually dimorphic (as in H. armiger; Zhang et al. 2020), indicating that olfactory signals play a vital role in mate choice and copulation (Muñoz-Romo et al. 2021). Moreover, we previously showed that male H. armiger uses olfactory signals to discriminate among individuals and also to mitigate the costs of conflict (Zhang et al. 2020). Thus, these results suggested that bats’ olfactory signals also served a role in agonistic interactions.
In summary, our study demonstrates that territorial calls and olfactory signals can be used as non-redundant elements of multimodal signals, and that acoustic components of multimodal signals play a more dominant role in territorial conflict of male H. armiger than olfactory components. To our knowledge, our study provides the first experimental evidence that acoustic signals dominate over olfactory signals during agonistic interactions in a nocturnal mammal. A limitation of this study is that we focused only on the response of receivers to a single level of each unimodal signal and on a single level of the multimodal signal. Additionally, we used a physiological response that is likely to be stress-based as an index of information content of the signals. Future studies are needed to determine the dynamic response of receivers to the interaction between multimodal signals of different magnitude and future studies should also evaluate aspects of receiver responses beyond an autonomic stress response.
Author Contributions
C.-M.Z., J.F., and T.-L.J. participated in the study design. C.-M.Z. and C.-N.S. collected the data. C.-M.Z. and C.-N.S. analyzed the data and wrote the manuscript. J.-R.L., J.F., and T.-L.J. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Ethical Statement
Animal husbandry and experimental procedures adhered to the Guidelines for the Use of Animals in Research (ASAB/ABS, 2021), and to the National Natural Science Foundation of China for experiments involving vertebrate animals, and were approved by the Northeast Animal Research Authority of Northeast Normal University, China (approval number: NENU-W-2008-108). There were no deaths during the whole surgery period. After completing the experiments, all 8 bats were returned to their original cave.
Supplementary Material
Acknowledgments
We thank Hao Gu for his help in the odors collection. We are grateful to Professor Brock Fenton and 2 anonymous reviewers for providing valuable comments on the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 31872680, 31922050) and the Program for Introducing Talents to Universities (B16011).
Supplementary Material
Supplementary material can be found at https://academic.oup.com/cz.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
Contributor Information
Chun-Mian Zhang, Jilin Provincial Key Laboratory of Animal Resource Conservation and Utilization, Northeast Normal University, 2555 Jingyue Street, Changchun 130117, China; Key Laboratory of Vegetation Ecology of Education Ministry, Institute of Grassland Science, Northeast Normal University, 2555 Jingyue Street, Changchun 130117, China.
Cong-Nan Sun, Jilin Provincial Key Laboratory of Animal Resource Conservation and Utilization, Northeast Normal University, 2555 Jingyue Street, Changchun 130117, China; Key Laboratory of Vegetation Ecology of Education Ministry, Institute of Grassland Science, Northeast Normal University, 2555 Jingyue Street, Changchun 130117, China.
Jeffrey R Lucas, Department of Biological Sciences, Purdue University, West Lafayette, IN 47907, USA.
Jiang Feng, Jilin Provincial Key Laboratory of Animal Resource Conservation and Utilization, Northeast Normal University, 2555 Jingyue Street, Changchun 130117, China; Key Laboratory of Vegetation Ecology of Education Ministry, Institute of Grassland Science, Northeast Normal University, 2555 Jingyue Street, Changchun 130117, China; College of Life Science, Jilin Agricultural University, 2888 Xincheng Street, Changchun 130118, China.
Ting-Lei Jiang, Jilin Provincial Key Laboratory of Animal Resource Conservation and Utilization, Northeast Normal University, 2555 Jingyue Street, Changchun 130117, China; Key Laboratory of Vegetation Ecology of Education Ministry, Institute of Grassland Science, Northeast Normal University, 2555 Jingyue Street, Changchun 130117, China.
References
- Arnott G, Elwood RW, 2009. Assessment of fighting ability in animal contests. Anim Behav 77:991–1004. [Google Scholar]
- Bayani DM, Taborsky M, Frommen JG, 2017. To pee or not to pee: urine signals mediate aggressive interactions in the cooperatively breeding cichlid Neolamprologus pulcher. Behav Ecol Sociobiol 71:37. [Google Scholar]
- Briffa M, 2015. Agonistic signals: integrating analysis of functions and mechanisms. In: Irschick DJ, Briffa M, Podos J, editors. Animal Signaling and Function: An Integrative Approach. Hoboken (NJ): Wiley Blackwell, 141–167. [Google Scholar]
- Bro-Jørgensen J, 2010. Dynamics of multiple signalling systems: animal communication in a world in flux. Trends Ecol Evol 25:292–300. [DOI] [PubMed] [Google Scholar]
- Chabrolles L, Ben Ammar I, Fernandez MSA, Boyer N, Attia J. et al., 2017. Appraisal of unimodal cues during agonistic interactions in Maylandia zebra. Peer J 5:e3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HC, Lee LL, 2004. Temporal variations in the size and composition of formosan leaf-nosed bat Hipposideros terasensis colonies in central Taiwan. Zool Stud 43:787–794. [Google Scholar]
- Déaux ÉC, Clarke JA, Charrier I, 2015. Aggressive bimodal communication in domestic dogs Canis familiaris. PLoS ONE 10:e0142975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bò E, Gentili C, Cecchetto C, 2020. Human chemosignals and brain activity: a preliminary meta-analysis of the processing of human body odors. Chem Senses 45:855–864. [DOI] [PubMed] [Google Scholar]
- Elias DO, Kasumovic MM, Punzalan D, Andrade MCB, Mason AC, 2008. Assessment during aggressive contests between male jumping spiders. Anim Behav 76:901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faragó T, Townsend S, Range F, 2014. The information content of wolf (and dog) social communication. In: Witzany G, editor. Biocommunication of Animals. Dordrecht, The Netherlands: Springer, 41–62. [Google Scholar]
- Fernandez AA, Fasel N, Knörnschild M, Richner H, 2014. When bats are boxing: aggressive behaviour and communication in male Seba’s short-tailed fruit bat. Anim Behav 98:149–156. [Google Scholar]
- Fröhlich M, Sievers C, Townsend SW, Gruber T, van Schaik CP, 2019. Multimodal communication and language origins: integrating gestures and vocalizations. Biol Rev 94:1809–1829. [DOI] [PubMed] [Google Scholar]
- Frommen JG, 2020. Aggressive communication in aquatic environments. Funct Ecol 34:364–380. [Google Scholar]
- Gadziola MA, Grimsley JM, Faure PA, Wenstrup JJ, 2012. Social vocalizations of big brown bats vary with behavioral context. PLoS ONE 7:e44550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadziola MA, Shanbhag SJ, Wenstrup JJ, 2016. Two distinct representations of social vocalizations in the basolateral amygdala. J Neurophysiol 115:868–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty E, Clay Z, Hobaiter C, Zuberbühler K, 2014. Multi-modal use of a socially directed call in bonobos. PLoS ONE 9:e84738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot J, Kirk P, Gottfried J, 2020. Encoding fear intensity in human sweat. Phil Trans R Soc B 375:20190271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidovic A, Van Hedger K, Choi SH, Flowers S, Wardle M. et al., 2020. Quantitative meta-analysis of heart rate variability finds reduced parasympathetic cardiac tone in women compared to men during laboratory-based social stress. Neurosci Biobehav Rev 114:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebets EA, 2008. Seismic signal dominance in the multimodal courtship display of the wolf spider Schizocosa stridulans Stratton 1991. Behav Ecol 19:1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebets EA, Papaj DR, 2005. Complex signal function: developing a framework of testable hypotheses. Behav Ecol Sociobiol 57:197–214. [Google Scholar]
- Johnstone RA, 1996. Multiple displays in animal communication: ‘backup signals’ and‘multiple messages’. Phil Trans R Soc B 351:329–338. [Google Scholar]
- Jong ICD, Sgoifo A, Lambooij E, Korte SM, Blokhuis HJ. et al., 2000. Effects of social stress on heart rate and heart rate variability in growing pigs. Can J Anim Sci 80:273–280. [Google Scholar]
- Jordan NR, Cherry MI, Manser MB, 2007. Latrine distribution and patterns of use by wild meerkats: implications for territory and mate defence. Anim Behav 73:613–622. [Google Scholar]
- Konoshenko MY, Timoshenko TV, Plyusnina IZ, 2013. c-Fos activation and intermale aggression in rats selected for behavior toward humans. Behav Brain Res 237:103–106. [DOI] [PubMed] [Google Scholar]
- Kurta A, Baker RH, 1990. Eptesicus fuscus. Mammalian Species 356:1–10. [Google Scholar]
- Møller AP, Pomiankowski A, 1993. Why have birds got multiple sexual ornaments. Behav Ecol Sociobiol 32:167–176. [Google Scholar]
- Marchant JN, Mendl MT, Rudd AR, Broom DM, 1995. The effect of agonistic interactions on the heart rate of group-housed sows. Appl Anim Behav Sci 46:49–56. [Google Scholar]
- Martins EP, Ossip-Drahos AG, Vital García C, Zúñiga-Vega JJ, Campos SM. et al., 2018. Trade-offs between visual and chemical behavioral responses. Behav Ecol Sociobiol 72:189. [Google Scholar]
- Martinez M, Phillips PJ, Herbert J, 1998. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci 10:20–33. [DOI] [PubMed] [Google Scholar]
- Mercier S, Deaux EC, van de Waal E, Bono AEJ, Zuberbuhler K, 2019. Correlates of social role and conflict severity in wild vervet monkey agonistic screams. PLoS ONE 14:e0214640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowles SL, Jennions M, Backwell PRY, 2017. Multimodal communication in courting fiddler crabs reveals male performance capacities. R Soc Open 4:161093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Romo M, Page RA, Kunz TH, 2021. Redefining the study of sexual dimorphism in bats: following the odour trail. Mammal Rev 51:155–177. [Google Scholar]
- Narins PM, Hodl W, Grabul DS, 2003. Bimodal signal requisite for agonistic behavior in a dart-poison frog, Epipedobates femoralis. Proc Natl Acad Sci USA 100:577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuweiler G, 2000. The Biology of Bats. In: Covey E, editor. Translator. New York (NY): Oxford University Press. [Google Scholar]
- Partan S, Marler P, 1999. Communication goes multimodal. Science 283:1272–1273. [DOI] [PubMed] [Google Scholar]
- Partan SR, 2013. Ten unanswered questions in multimodal communication. Behav Ecol Sociobiol 67:1523–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partan SR, Larco CP, Owens MJ, 2009. Wild tree squirrels respond with multisensory enhancement to conspecific robot alarm behaviour. Anim Behav 77:1127–1135. [Google Scholar]
- Partan SR, Marler P, 2005. Issues in the classification of multimodal communication signals. Am Nat 166:231–245. [DOI] [PubMed] [Google Scholar]
- Polheber JP, Matchock RL, 2014. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med 37:860–867. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, 1992. Neuroendocrinology of the stress–response. In: Becker JB, Breedlove SM, Crews D editors. Behavioral Endocrinology. Cambridge (MA): MIT Press, 287–324. [Google Scholar]
- Semin G, Scandurra A, Baragli P, Lanatà A, D’Aniello B, 2019. Inter- and intra-species communication of emotion: chemosignals as the neglected medium. Animals 9:887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöhr W, 1986. Heart rate of tree shrews and its persistent modification by social contact. In: Schmidt TH, Dembroski TM, Blümchen G, editors. Biological and Psychological Factors in Cardiovascular Disease. Berlin, Heidelberg, Germany: Springer, 491–499. [Google Scholar]
- Stevens M, 2013. Sensory Ecology, Behavior and Evolution. Oxford: Oxford University Press. [Google Scholar]
- Stoddard SL, Bergdall VK, Townsend DW, Levin BE, 1986. Plasma catecholamines associated with hypothalamically-elicited flight behavior. Physiol Behav 37:709–715. [DOI] [PubMed] [Google Scholar]
- Stritih N, Kosi AŽ, 2017. Olfactory signaling of aggressive intent in male-male contests of cave crickets (Troglophilus neglectus; Orthoptera: rhaphidophoridae). PLoS ONE 12:e0187512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Jiang T, Kanwal JS, Guo X, Luo B. et al., 2018. Great Himalayan leaf-nosed bats modify vocalizations to communicate threat escalation during agonistic interactions. Behav Process 157:180–187. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang C, Gu H, Lucas JR, Feng J. et al., 2021. Vocal performance reflects individual quality in male Himalayan leaf-nosed bats (Hipposideros armiger). Integr Zool doi: 10.1111/1749-4877.12545. [DOI] [PubMed] [Google Scholar]
- Takahashi A, Flanigan ME, McEwen BS, Russo SJ, 2018. Aggression, social stress, and the immune system in humans and animal models. Front Behav Neurosci 12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeles EJ, 1994. The role of neighbours in territorial systems: when are they ‘dear enemies’? Anim Behav 47:339–350. [Google Scholar]
- Vannoni E, McElligott AG, 2008. Low frequency groans indicate larger and more dominant fallow deer Dama dama males. PLoS ONE 3:e3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher CAF, 2021. Heart rate as a measure of emotional arousal in evolutionary biology. Proc R Soc B 376:20200479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wascher CAF, Scheiber IBR, Weiß BM, Kotrschal K, 2009. Heart rate responses to agonistic encounters in greylag geese Anser anser. Anim Behav 77:955–961. [Google Scholar]
- Wierucka K, Pitcher BJ, Harcourt R, Charrier I, 2018. Multimodal mother—offspring recognition: the relative importance of sensory cues in a colonial mammal. Anim Behav 146:135–142. [Google Scholar]
- Wyatt TD, 2014. Pheromones and Animal Behavior: Chemical Signals and Signatures. Cambridge: Cambridge University Press. [Google Scholar]
- Yang YJ, 2011. Mating System and Kinship of the Formasan Leaf-Nosed Bat Hipposideros armiger Terasensis (Chiroptera, Hippsideridae). Dissertation. National Chung Hsing University, Taichung.
- Ydenberg RC, Giraldeau LA, Falls JB, 1988. Neighbours, strangers, and the asymmetric war of attrition. Anim Behav 36:343–347. [Google Scholar]
- Zhang C, Sun C, Lucas JR, Gu H, Feng J. et al., 2021. Individuality and function of chemical signals in conflict resolution of a mammal. Ann NY Acad Sci. In Press. doi: 10.1111/nyas.14712). [DOI] [PubMed]
- Zhang C, Sun C, Wang Z, Lin P, Xiao Y. et al., 2021. Minor modifcation of frequency modulated call parameters underlies a shift in behavioral response in the Great Himalayan leaf-nosed bats Hipposideros armiger. J Mammal 102:457–467. [Google Scholar]
- Zhu B, Zhou Y, Yang Y, Deng K, Wang T. et al., 2021. Multisensory modalities increase working memory for mating signals in a treefrog. J Anim Ecol 90:1455–1465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.