Figure 3.
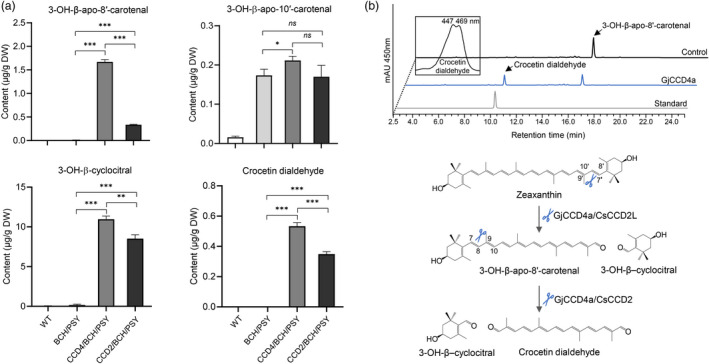
Analysis of hydroxylated apocarotenoids and crocetin dialdehyde in different engineered callus lines and in vitro assay of GjCCD4a with the C30 3‐OH‐β‐apo‐8′‐carotenal substrate. (a) Relative quantification of hydroxylated apocarotenoids among various engineered callus lines. Column names are defined in the legend of Figure 1. Analysis of hydroxylated apocarotenoids of other independent lines is shown in Figure S6. (b) UHPLC analysis of GjCCD4a activity and cleavage reactions for crocetin dialdehyde biosynthesis from 3‐OH‐β‐apo‐8′‐carotenal. Separation was performed in UHPLC system 2. The same HPLC‐DAD chromatogram of crocetin dialdehyde standard was also shown in Figure 2. The insets show UV/vis spectrum of GjCCD4a product crocetin dialdehyde.
