FIG 3.
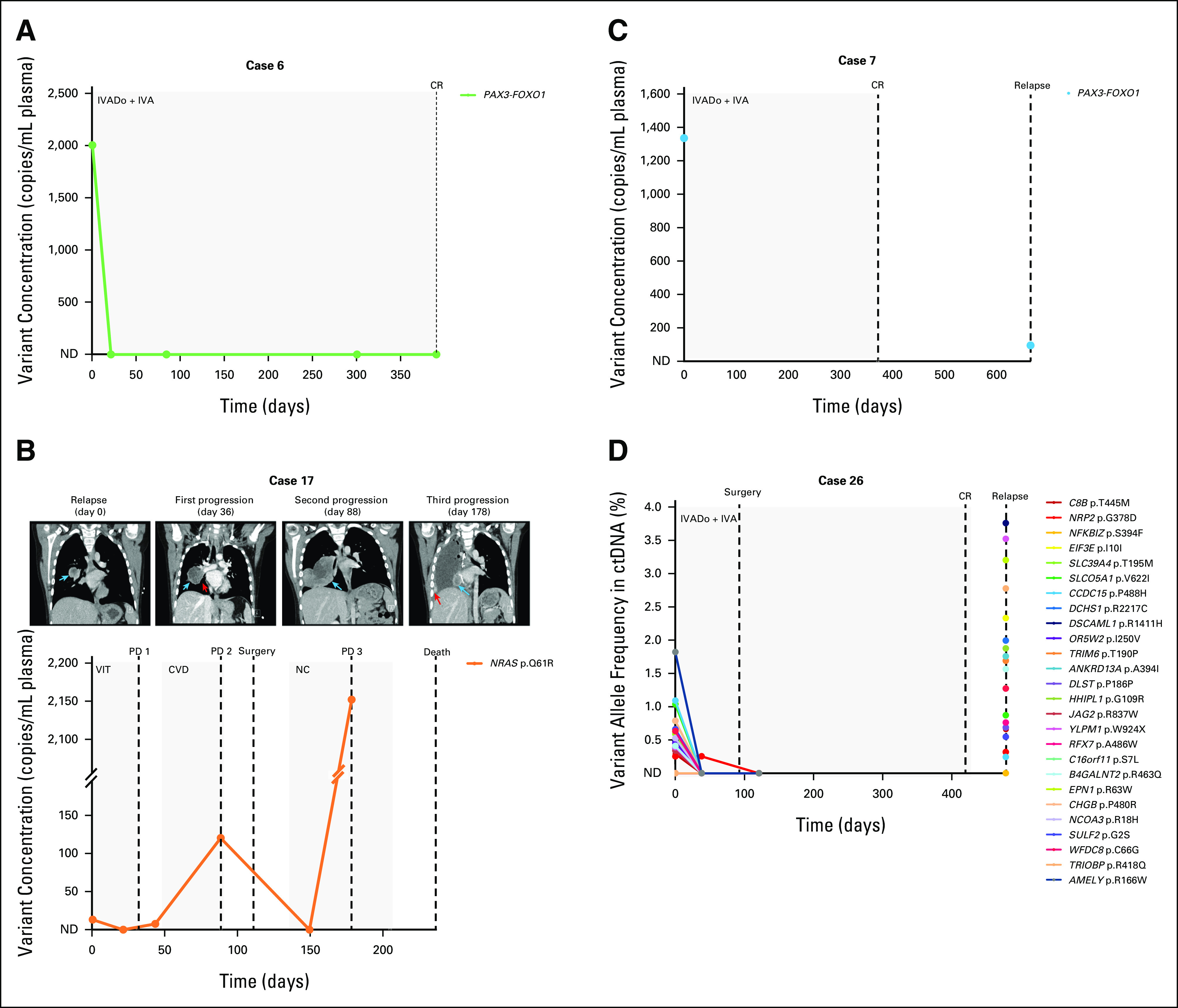
Patient ctDNA levels reflect disease burden over time. (A) A PAX3-FOXO1 rearrangement in the pretreatment ctDNA of a frontline patient with aRMS became undetectable via ddPCR in plasma samples collected during chemotherapy. The patient ended therapy with a complete response. (B) Plasma levels of an NRAS variant in a relapsed eRMS patient with pulmonary metastasis (day 0 CT image, blue arrow) initially decreased after initiation of chemotherapy but increased as the patient's neoplasm enlarged (see enlarged nodule indicated by blue arrow and narrowing of right lower bronchus indicated by red arrow, CT images at days 36 and 88, respectively). The patient was deemed to have disease progression according to the RECIST 1.1.24 Following surgery and adjuvant chemotherapy, ctDNA became undetectable via ddPCR, but a subsequent plasma sample illustrated a re-emergence of the variant, coinciding with further progression in the patient (new pulmonary metastasis in the surgical bed denoted by blue arrow and scattered vascularized ipsilateral pleural deposits indicated by red arrow in CT image day 178; note the broken y-axis of the graph). The patient died 2 months later. (C) A PAX3-FOXO1 fusion initially identified in the pretreatment ctDNA of a patient with frontline aRMS was also detected in a ctDNA sample collected at time of relapse via ddPCR, albeit at a lower concentration. (D) Targeted sequencing of cfDNA from an aRMS patient illustrates an initial response to frontline treatment, as evidenced by decreasing variant allele frequencies (%) in serial plasma samples. However, ctDNA was detected in a blood sample collected 2 months after the completion of treatment, coinciding with clinical relapse. Day 0 for all patients is the day that the pretreatment blood sample was collected. Dots on the line graph correspond to the days in which plasma samples were obtained. Gray boxes indicate the chemotherapy duration. Dashed lines indicate clinical time point (surgery, response as assessed on imaging). cfDNA, cell-free DNA; CR, complete response; CT, computed tomography; ctDNA, circulating tumor DNA; CVD, cyclophosphamide, vincristine, and doxorubicin; ddPCR, droplet digital polymerase chain reaction; eRMS, embryonal RMS; IVA, ifosfamide, vincristine, and actinomycin D; IVADo, ifosfamide, vincristine, actinomycin D, and doxorubicin; NC, navelbine (vinorelbine) and cyclophosphamide; ND, not detected; PD, progressive disease; RMS, rhabdomyosarcoma; VIT, vincristine, irinotecan, and temozolomide.
