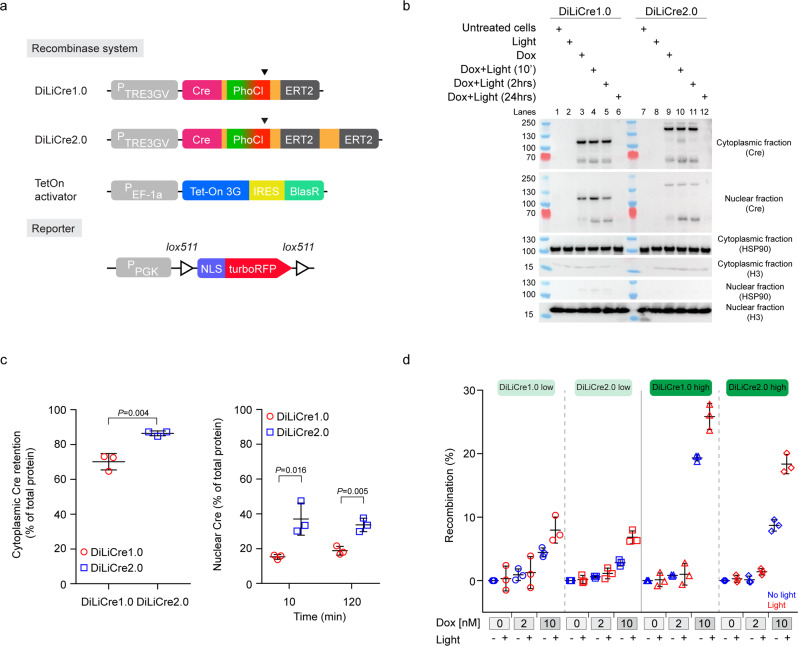
Fig. 2. Modifications in DiLiCre2.0 reduce the background recombination whilst maintaining the efficiency of light induction.
a Schematic representation of DiLiCre1.0 and DiLiCre2.0 constructs and the floxed nuclear-RFP cassette. Arrowheads the light-breaking points of the PhoCl fluorophore. b Representative nuclear-cytoplasmic protein fractionation time course experiment carried out in DiLiCre1.0 and DiLiCre2.0 HEK293T cells. Four different conditions were tested: untreated cells, cells exposed to doxycycline or light, and combined treatment (10 nM dox and 0.5 mW/mm2 90-s-light pulse). Three time points were compared (10 min, 2 h, and 24 h). n = 3 biologically independent experiments were performed with similar results. Protein molecular weights (kDa) are annotated on the left side of the scans. c Protein densitometries from the nuclear-cytoplasmic assays. Plots represent the percentage of total Cre retained in the cytoplasm (left graph) or delivered in the nucleus (right graph). All percentages were normalized by the total Cre protein content. Data represents the mean ± SD from n = 3 biologically independent experiments. P values were calculated by a two-tailed t-test. d FACS quantification of the light-mediated recombination in HEK293T cells stably expressing the nuclear RFP reporter and sorted for low and high expression of DiLiCre1.0 or 2.0 constructs. All sorted populations were tested for two different concentrations of doxycycline (2 or 10 nM). For each treatment, cells were both exposed and non-exposed to light (0.5 mW/mm2 30 s pulses every 4 h [x3]). Control values (untreated cells) were subtracted. Data represents the mean ± SD from n = 3 biologically independent experiments. Source data are provided as a Source Data file.