Fig.3 |. Chemo-and regioselectivity study of aryl halide/18F interconversion.
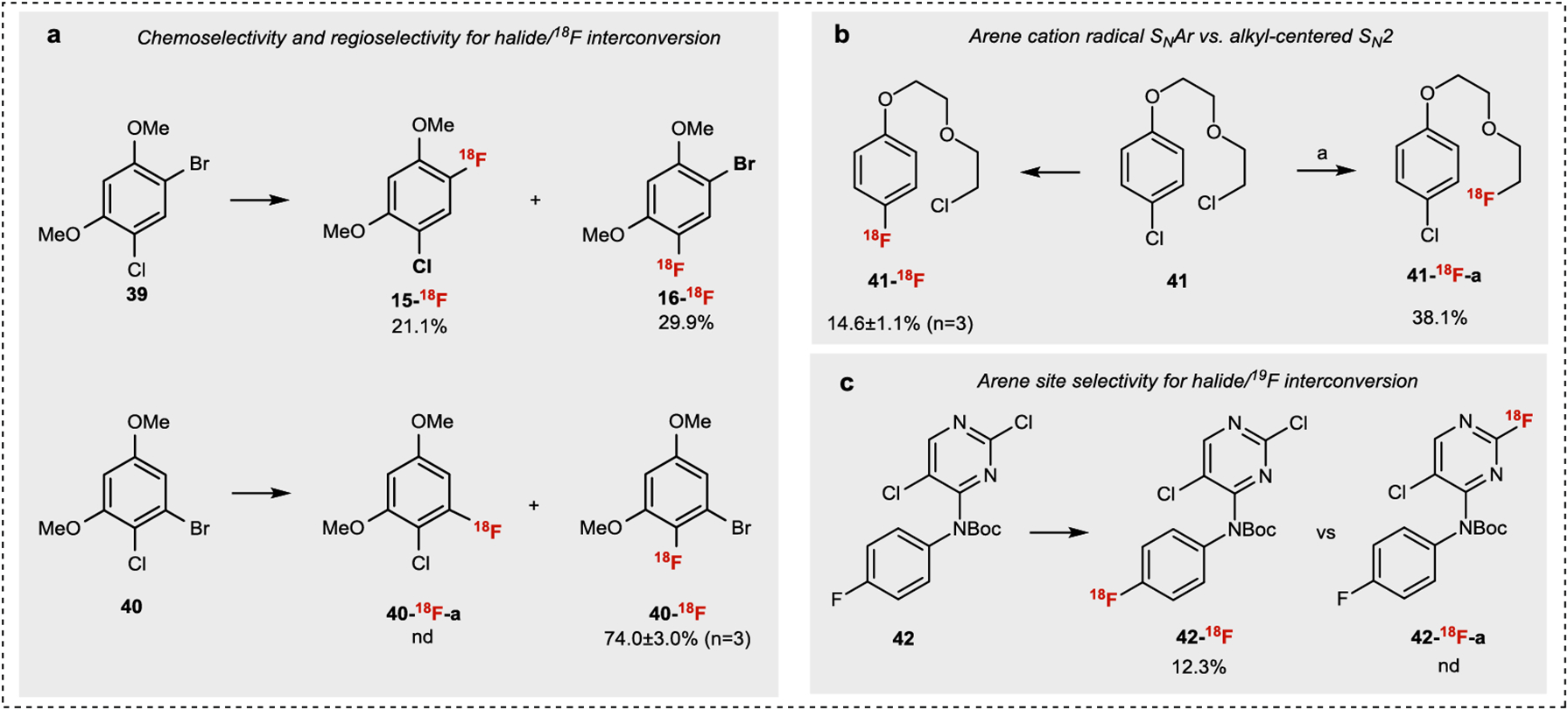
All halide/18F interconversion reactions were conducted under standard conditions listed in Fig.2. a. Comparison of reactivity between ArBr and ArCl. b. Comparison of reactivity between SNAr and SN2 under light condition. c. Comparison of reactivity between electron rich and electron deficient aromatic rings. a[18F]TBAF, MeCN, 100°C, 10 min (See SI for details).
