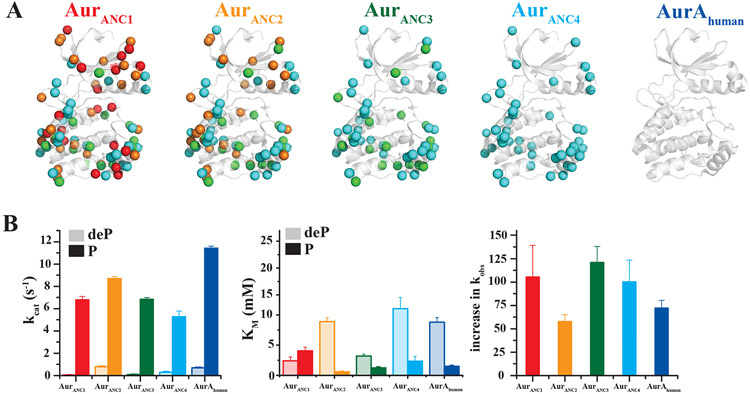
Fig. 2. Autophosphorylation is the oldest and uniformly conserved allosteric activation mechanism in all Aurora kinases.
(A) Additive differences in primary sequence between AurAhuman and AurANC4 (82.4% identity), AurANC3 (80.1% identity), AurANC2 (72.7% identity) and AurANC1 (69.5% identity) are shown in cyan, green, orange and red spheres, respectively. (B) Corresponding kcat (left) and KM (middle) for dephosphorylated (deP) and T288-phosphorylated (P) Aurora kinases show that phosphorylation increases catalytic efficiency by increasing kcat and decreasing KM (see also Fig. S4). To best illustrate this combined effect, the fold increase in kobs at 1 mM Lats2 upon Aurora’s T288 phosphorylation is shown on the right. Phosphorylation of Lats2 peptide was monitored using the ADP/NADH coupled assay with 1 μM dephosphorylated or 0.05 μM phosphorylated Aurora and 5 mM ATP, 20 mM MgCl2 at 25°C. Error bars represent the standard error for the estimate of kcat or KM through the Michaelis-Menten equation and are a measure of the goodness of fit of the data. Error bars for increase in kobs are calculated using jackknifing and error propagation.