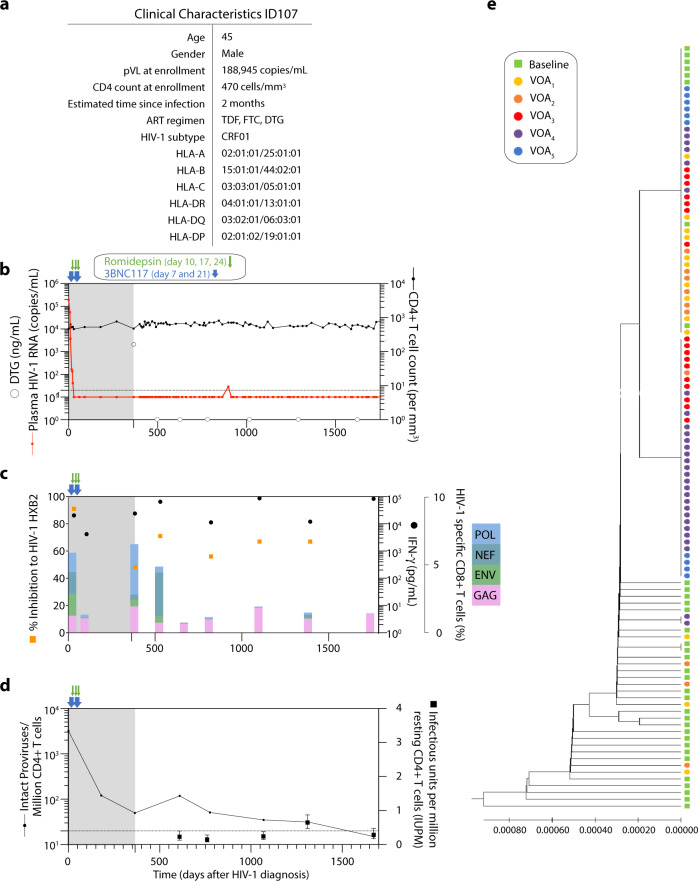
Fig. 5. Clinical and immunological characteristics of ID107.
a Clinical characteristics for ID107: age, gender, plasma viral load and CD4 count at enrollment, estimated time since infection, ART regimen, HIV-1 subtype and HLA typing. b Plasma HIV-1 RNA levels (copies/mL) and CD4+ T cell counts are shown longitudinally over the period of the interventional (gray area) and ART-free (white area) period. Time points for measurement of the concentration of dolutegravir (DTG) (ng/mL) shown as gray circles over time. c Frequency of HIV-1-specific CD8+ T cell responses to HIV-1 Env, Gag, Pol and Nef detected by the AIM assay shown as color coded in stacked bars. IFN-γ concentration following Gag stimulation shown in black dots and percentage of viral inhibition against HIV-1HXB2 shown in orange squares. d Number of intact proviruses detected per 106 CD4+ T cells (black line) and infectious units per million resting CD4+ T cells (mean and 95% CI, black squares). e UPGMA phylogenetic tree of plasma baseline env sequences (green) and viral outgrowth from five different time points (VOA1, VOA2, VOA3, VOA4 and VOA5 at days 610, 776, 1066 1311 and 1674 after ART initiation, respectively) using Jukes-Cantor model. Source data are provided as a Source Data file.