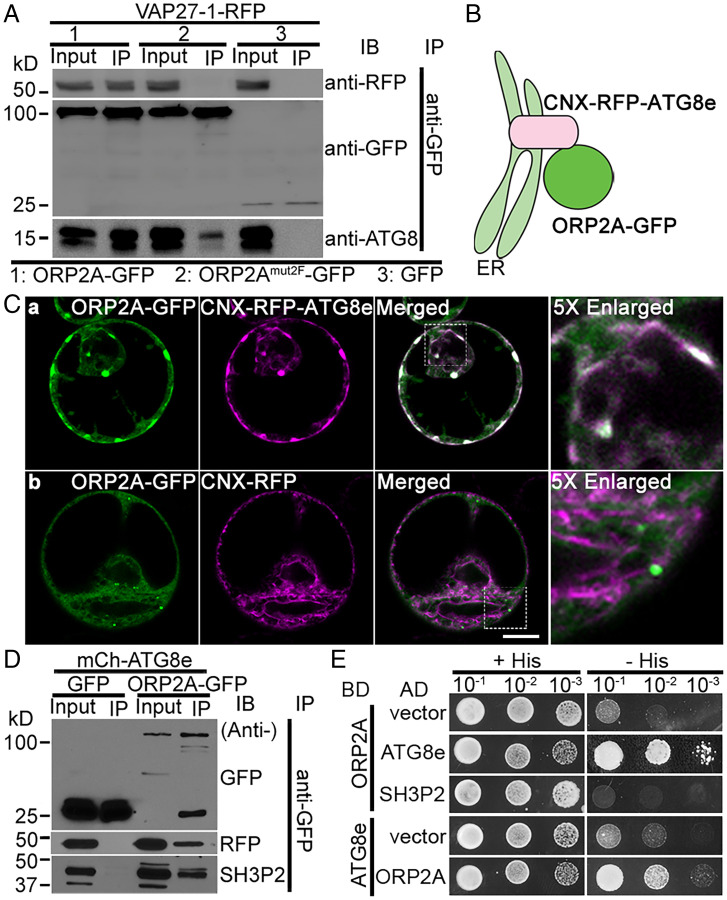
Fig. 3.
ORP2A interacts directly with both ATG8e and VAP27-1. (A) ORP2A interacted with VAP27-1 in an IP assay. Cell lysates (input) prepared from Arabidopsis PSBD protoplasts transiently coexpressing VAP27-1-RFP with ORP2A-GFP/ORP2Amut2F-GFP/GFP (control) (Fig. 2B) were subjected to GFP-Trap assays and subsequent immunoblotting (IB) analysis using GFP, RFP, or ATG8 antibodies as indicated. (B) Working principle of the CNX recruitment assay. RFP-ATG8e was conjugated with the ER transmembrane protein CNX, resulting in ER localization of CNX-RFP-ATG8e with its C terminus facing the cytoplasm, thus recruiting ORP2A to the ER upon their coexpression because of ATG8e–ORP2A interaction. (C) ORP2A-GFP was recruited to the ER membrane by CNX-RFP-ATG8e. ORP2A-GFP was transiently coexpressed with CNX-RFP-ATG8e (a) or CNX-RFP (b, control), respectively, in Arabidopsis protoplasts, followed by confocal imaging. (Scale bar, 10 μm.) (D) ORP2A interacted with ATG8e and SH3P2 in IP assays. Cell lysates prepared from Arabidopsis PSBD protoplasts transiently coexpressing mCh-ATG8e/GFP (control) or mCh-ATG8e/ORP2A-GFP were subjected to GFP-Trap assays and subsequent IB analysis using GFP, RFP, or SH3P2 antibodies as indicated. (E) Yeast two-hybrid analysis of the binary interactions between ORP2A and ATG8e or SH3P2. AD, activation domain; BD, binding domain.