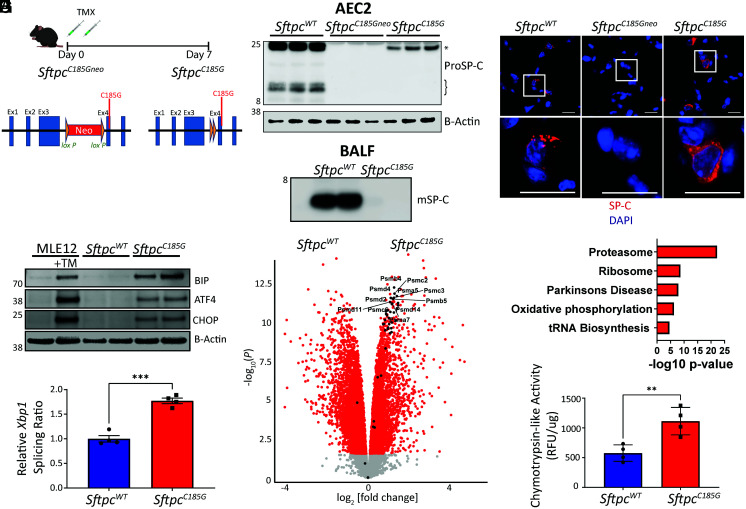
Fig. 1.
Expression of a clinical Sftpc mutation challenges AEC2 proteostasis and activates the UPR. (A) Schematic of SftpcC185Gneo founder line with LoxP-flanked inhibitory neomycin cassette and post-TMX induced Cre recombination to the active SftpcC185G. (B) (Top) Western blotting of AEC2 cell lysate 7 d post-TMX for SP-C shows primary translation product (*) in SftpcWT and SftpcC185G samples, an absence of SP-C in the SftpcC185Gneo founder line, and a failure of SftpcC185G SP-C to undergo proteolytic cleavage to intermediate isoforms (}). (Bottom) Western blotting of BALF large-aggregate surfactant fractions for mature SP-C (mSP-C) shows absence of mSP-C in alveolar compartment of SftpcC185G mice (n = 2 per genotype). (C) Representative immunostaining of lung section for SP-C shows punctae appearance in SftpcWT, an absence of SP-C in the SftpcC185Gneo founder line, or a reticular pattern to the SftpcC185G SP-C (60× magnification, scale bars = 20 μM). (D) Western blotting analysis for BIP, ATF4, and CHOP in MLE12 cells ± 500 nm tunicamycin (TM) and SftpcWT and SftpcC185G AEC2 lysates 7 d post-TMX. (E) Ratio of spliced to unspliced Xbp1 as assessed by qRT-PCR of SftpcWT and SftpcC185G AEC2s. (F) Volcano plot showing the differences in gene expression in SftpcWT versus SftpcC185G AEC2s (red = >1.5 fold change, adj P value < 0.05). Genes annotated to the proteasome are indicated and show enrichment in SftpcC185G AEC2s. (G) Kyoto Encyclopedia of Genes and Genomes pathway analysis of differentially expressed genes shows enrichment of the proteasome pathway in SftpcC185G AEC2s. (H) Proteasome activity assessed as chymotrypsin-like activity in cell lysates of AEC2 isolated 7 d post-TMX. **p < 0.005, ***p < 0.0005 by two-way t test.