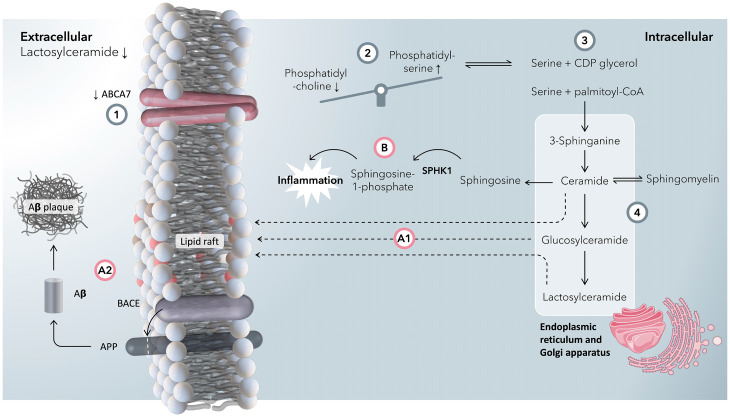
Fig. 7.
A summary graphic of the proposed mechanism by which ABCA7 dysfunction is associated with an increased risk of developing Alzheimer’s disease. (1) A reduction in expression/activity of ABCA7 is associated with an increase in AD risk, as well as a reduction in blood-plasma concentrations of lactosylceramides. In our putative mechanism, we rationalize this with a reduction in the flux of lipids through ABCA7. (2) ABCA7 has more affinity to export phosphatidylserines (PSs) than phosphatidylcholines (PCs) across the plasma membrane compared with other ABCA lipid transporters (e.g., ABCA1) (25). With a reduction in the function of ABCA7, intracellular concentrations of PSs increase relative to PCs. (3) As PSs accumulate intracellularly, so does serine (PS is broken down to CDP-glycerol and serine). Serine and palmitoyl-coenzyme A (CoA) form 3-sphinganine at the start of the ceramide synthesis pathway. (4) In the ceramide pathway, 3-sphinganine is synthesized in the ER, which after some steps is converted into ceramides and, in subsequent steps, glucosylceramides and lactosylceramides in the Golgi apparatus. At this stage, there is a branch point. (A1) Ceramides, glucosylceramides, and lactosylceramides can all form lipid rafts (39) in the plasma membrane. (A2) Lipid rafts encourage interactions between β-site APP cleaving enzyme 1 (BACE1) and amyloid-β precursor protein (APP) to make amyloid-β, which ultimately forms plaques (13). (B) Ceramides are broken down to sphingosine in the ER, a precursor for sphingosine-1-phosphate via SPHK1, both of which are increased in inflammation as well as induce inflammation in a feedforward step. Because of disrupted PS metabolism and a failure in exporting PS to the extracellular space, blood concentrations of lactosylceramide decrease.