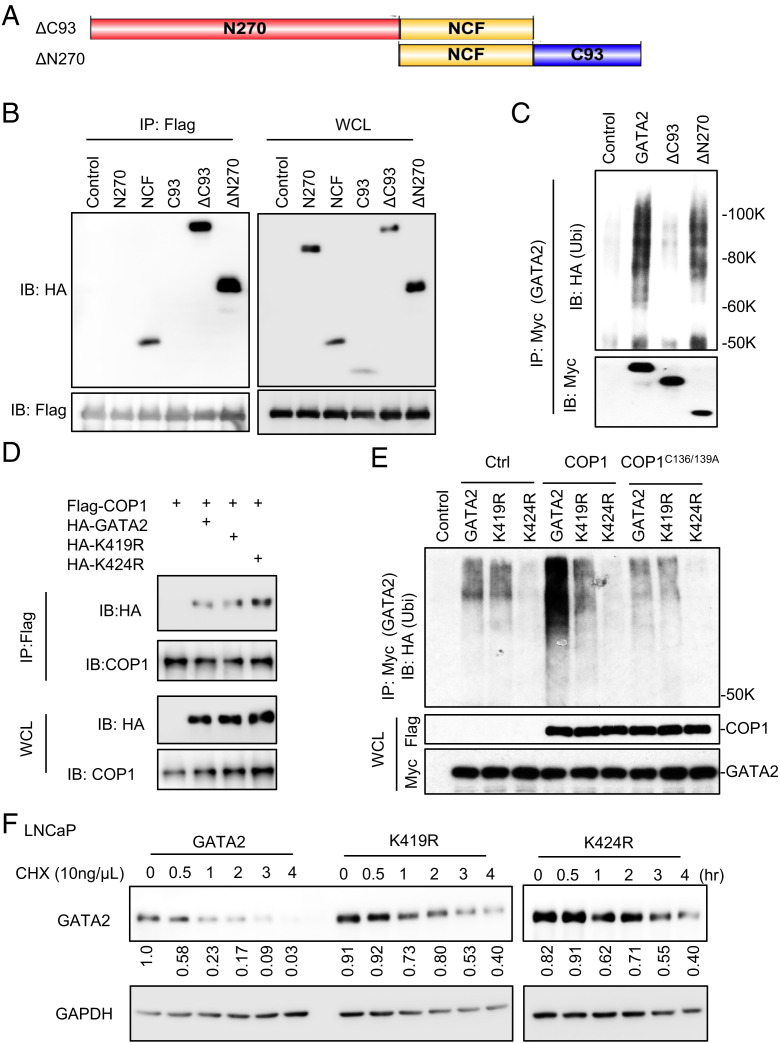
Fig. 5.
COP1 ubiquitinates GATA2 at lysines 419 and 424. (A) Illustration of the ΔC93 (deletion of C93) and ΔN270 (deletion of N270) fragments of GATA2. (B) HEK293T cells were transfected with Flag-COP1 and HA-tagged GATA2 truncated, including N270, NCF, C93, ΔC93, and ΔN270 as shown in A and Fig. 3G. After 48 h, the cells were treated with 10 μM MG132 for 6 h before preparing the cell lysate for IP using anti-Flag affinity gel and IB analysis. (C) Ubiquitination assay. Myc-tagged GATA2 or its truncations ΔC93 or ΔN270 were transfected into HEK293T cells along with HA-Ubi. After 48 h, cells were treated with 10 μM MG132 for 6 h before preparing the cell lysate for IP using anti-Myc antibody and IB analysis. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. (D) HEK293T cells were transfected with Flag-COP1 and HA-tagged GATA2 or GATA2 mutants K419R or K424R. After 48 h, cells were treated with 10 μM MG132 for 6 h before preparing the cell lysate for IP using anti-Flag affinity gel and IB analysis. (E) Ubiquitination assay. Myc-tagged GATA2 versus GATA2 mutants K419R or K424R and COP1 versus Ctrl or COP1C136/139A mutant was transfected into HEK293T cells along with HA-Ubi. After 48 h, cells were treated with 10 μM MG132 for 6 h before preparing the cell lysate for IP using anti-Myc antibody and IB analysis. (F) LNCaP cells with 0.5 µg/mL Dox-induced GATA2 or GATA2 mutant K419R or K424R expression were treated with 10 ng/µL chlorhexidine (CHX) for 0–4 h. GAPDH, glyceraldehyde 3-phosphate dehydrogenase. Data shown are Western blots and are representative of at least three independent experiments. Western blots in panel (F) were also quantified using Image J; WCL, whole cell lysate.