Abstract
Previous research using functional MRI identified brain regions associated with sensory processing sensitivity (SPS), a proposed normal phenotype trait. To further validate SPS, to characterize it anatomically, and to test the usefulness in psychology of methodologies that assess axonal properties, the present study correlated SPS proxy questionnaire scores (adjusted for neuroticism) with diffusion tensor imaging (DTI) measures. Participants (n = 408) from the Human Connectome Project were studied. Voxelwise analysis showed that mean- and radial diffusivity correlated positively with SPS scores in the right and left subcallosal and anterior–ventral cingulum bundle, and the right forceps minor of the corpus callosum, all frontal cortex areas generally underlying emotion, motivation, and cognition. Further analyses showed correlations throughout medial frontal cortical regions in the right and left ventromedial prefrontal cortex, including the superior longitudinal fasciculus, inferior fronto-occipital fasciculus, uncinate, and arcuate fasciculus. Fractional anisotropy was negatively correlated with SPS scores in white matter (WM) of the right premotor/motor/somatosensory/supramarginal gyrus regions. Region of interest (ROI) analysis showed small effect sizes (− 0.165 to 0.148) in WM of the precuneus and inferior frontal gyrus. Other ROI effects were found in the dorsal-, ventral visual pathways and primary auditory cortex. The results reveal that in a large group of participants, axonal microarchitectural differences can be identified with SPS traits that are subtle and in the range of typical behavior. The results suggest that the heightened sensory processing in people who show that SPS may be influenced by the microstructure of WM in specific cortical regions. Although previous fMRI studies had identified most of these areas, the DTI results put a new focus on brain areas related to attention and cognitive flexibility, empathy, emotion, and first levels of sensory processing, as in primary auditory cortex. Psychological trait characterization may benefit from DTI methodology by identifying influential brain systems for traits.
Keywords: Diffusion MRI, Diffusion tensor imaging, Mean diffusivity, Highly sensitive people, Sensory processing sensitivity, Cingulum microstructure
Introduction
Sensory processing sensitivity
Sensory processing sensitivity (SPS) (Aron and Aron 1997) is proposed to be a normal phenotype trait observable as a high degree of environmental sensitivity (Pluess 2015). It is unrelated to sensory processing disorder. Heritability explains about 42% of the variance in twin studies (Assary et al. 2020). The trait is present in about 20% of the population (Lionetti et al. 2018); the estimated number varies from 15 (Kagan 1997) to 30% (Pluess et al. 2018). A similar proportion is found in many other species, suggesting the presence of two major survival strategies (Wolf et al. 2008): (1) SPS: A high level of attention to environmental stimuli and observing carefully before acting and (2) a normal level of attention to the environment and thus being the first to act. High attention to the environment appears only in a minority of a population, because this high sensitivity benefits an individual only if the majority of members of the species lack it (negative frequency dependence).
In humans, this increased sensitivity compared to the general population is hypothesized to result from a greater depth of processing of sensory input, e.g., using many cognitive tags (Aron and Aron 1997; Aron et al. 2012). Lockhart et al. (1976) suggested that memory is enhanced by processing information to deeper cognitive levels using a letter, word, word in context, or word abstracted from many contexts, a concept important to educational methodology (Leow 2018). This type of processing, using semantics or many contexts to remember a visual stimulus, is thought to enhance awareness of subtle stimuli and increase emotional responsiveness to stimuli in people with SPS. The emotional responsiveness may in turn act as a motivator for the depth of processing (Baumeister et al. 2007).
Research on SPS is growing; for a review, see (Greven et al. 2019). For example, research using fMRI found that SPS was associated with significantly greater activation in brain areas involved in higher order visual processing, which is more evidence for greater depth of processing in SPS compared to the general population. However, there are many open questions, especially about neuroanatomical correlates. There has been no previous research that investigates microstructural changes using diffusion tensor imaging or similar techniques.
SPS does not appear to be a disorder, given the percentages in the population, its presence in many species, and its functionality as a successful survival strategy. In a review comparing fMRI studies of SPS (Acevedo et al. 2018), autism spectrum disorder, schizophrenia, and post-traumatic stress disorder, the authors conclude that SPS engages brain regions differently from these disorders, namely those that are involved in reward processing, memory, physiological homoeostasis, self-other processing, empathy, and awareness. However, SPS can be related to disorders in that it leads to greater susceptibility to environmental influences (Belsky and Pluess 2009). For example, adults high on SPS scores who report difficult childhoods are more prone to depression, anxiety, and shyness (Aron et al. 2005); however, young children who exhibit SPS and had especially good childhood environments performed well on measures of social and academic competence (Pluess and Belsky 2010), while those with worse environments fared poorly.
The trait also has been shown to lead to greater positive outcomes following interventions (Pluess and Boniwell 2015; Nocentini et al. 2018), again suggesting that scores on the measure are correlated with greater susceptibility to environmental influences. For example, a study (Karam et al. 2019) of Syrian refugee children suggests that high-SPS scorers’ previous experience with family trauma (abuse, neglect, etc.) appeared to prepare them to be less affected by war trauma, while those with positive family histories reported more trauma from similar war-related events, again suggesting a survival strategy of noticing more rather than simply needing a positive childhood to adjust well to any circumstance. Differential susceptibility makes the sample in the present study particularly valuable given that participants were screened to avoid those with psychological disorders. Axonal microstructural differences possibly due to a seriously problematic past would not confound our results.
The Young Adult-Human Connectome Project
The dataset of the Young Adult Human Connectome Project (YA-HCP) offered a large group to analyze for a normal psychological variable and any relationship it might have to axonal microarchitectural measures. This open data cohort includes high-quality imaging data and an extensive range of data analysis options. There is also extensive psychological testing for each participant.
Proxy scale used to measure SPS
The questionnaire measure for SPS used in previous studies is called the HSP or Highly Sensitive Person Scale (Aron and Aron 1997). The YA-HCP testing does not include the HSP Scale and it was not feasible to re-contact participants to administer it. However, the YA-HCP dataset does include a substantial number of multi-item self-report personality measures. Thus, it was possible to identify a subset of items in the YA-HCP dataset that could serve as a proxy measure. As described in the methods, we developed and validated in other groups a 17-item proxy scale. We call this scale that measures SPS in the present report the neuroticism-adjusted residual proxy HSP scale, or Proxy HSP Scale.
Microstructural characteristics: diffusion tensor imaging
Microstructural characteristics associated with SPS would be another strong piece of evidence that it is a significant, reliable psychological trait. Also, identification of brain regional effects contributes to better understanding of SPS functional systems. Thus, we used diffusion tensor imaging (DTI) to identify any possible axonal microstructural characteristics for SPS. We captured measures from mean diffusivity (MD), radial diffusivity (RD), axial diffusivity (AD) and fractional anisotropy (FA). First, we used an exploratory voxelwise analysis for the whole brain. In addition, we used fMRI data from previous studies to carry out a region of interest (ROI) analysis.
Previous fMRI studies
Several fMRI studies helped to validate SPS as a psychological trait affecting sensory processing by finding correlations between the standard self-report measure of SPS and neurophysiological events during a variety of perceptual tasks. These studies provided the locations for the ROI analysis. The first study used a task of perceiving subtle differences in neutral landscapes (Jagiellowicz et al. 2011). When detecting minor (vs. major) changes in the landscape, high scores on the standard HSP Scale were associated with greater activation in brain areas involved in higher order visual processing: left occipitotemporal, bilateral temporal, and medial and posterior parietal regions.
Another fMRI study looked at culturally influenced visual perception (Hedden et al. 2008). The researchers gave 10 European–Americans and 10 East-Asians a visuo-spatial task that was either context independent (judging the length of a line independent of a surrounding box, the absolute condition, typically harder for Asians) or context dependent (judging the length of a line while paying attention to the box, the relative condition, typically harder for Americans). Each group exhibited greater activation for the culturally non-preferred task in frontal and parietal regions associated with greater effort in attention and working memory. In the two cultural groups, the HSP scale scores moderated the brain activations, such that neither cultural group with high HSP scores showed greater activation on their culturally more difficult task (Aron et al. 2010). The data suggest that the high-SPS participants were processing both the relative and absolute conditions, unaffected by their culture, by paying close attention to details of the stimulus.
Another fMRI study used visual stimuli that were photos of familiar or unfamiliar faces with happy, neutral, or sad expressions (Acevedo et al. 2014). Across all conditions, standard HSP scores were associated with increased brain activation of regions involved in attention and action planning (in the cingulate and premotor area (PMA). For happy- and sad-face conditions, SPS was associated with activation of brain regions involved in self-awareness, integration of sensory information, empathy, and action planning (e.g., cingulate, insula, inferior frontal gyrus [IFG], middle temporal gyrus [MTG], and premotor area [PMA]).
Finally, SPS individuals showed substantial differences compared to others in brain activation in response to emotional (versus neutral) images (nonsocial visual International Affective Picture System images; Acevedo et al. 2017). Standard HSP scores were associated with neural activations in the temporal/parietal area and areas that process emotional memory, learning, awareness, reflective thinking, and integration of information. There were similar results in the same study for an SPS × Quality of Childhood Parenting (QCP) interaction. For positive stimuli, SPS showed significant correlations with activation in subcortical areas involved in reward processing, self-other integration (insula and IFG), calm (PAG), and satiation (subcallosal AC). These were stronger with increasing QCP. For negative stimuli, the SPS × QCP interaction showed significant activation in the amygdala and prefrontal cortex (PFC) involved in emotion and self-control.
Overall, these fMRI studies show that SPS is associated with greater activation in multiple brain areas when processing subtle visual differences in neutral stimuli as well as stimuli evoking emotion or empathy and personally relevant social stimuli. The ROIs for this study were in areas whose activation was correlated with SPS under these conditions.
Study aims
We undertook this study to further validate and contribute to the broad understanding of SPS as an innate trait associated with a high level of perceptual attention. This trait accounts for a broadly defined attentional survival strategy: high-level attention to detail. In the present research, we correlated HSP-proxy questionnaire scores with DTI measures to assess any axonal microarchitecture measures associated with SPS, particularly in brain areas that might be related to primary and secondary perceptual processes. Importantly, we also wanted to determine if a subtle behavioral trait such as SPS could be detected using DTI. The results suggest that the heightened sensory processing in people with the SPS trait may be influenced by the anatomical microstructure of white matter in specific neocortical regions. Although previous fMRI studies had identified most of these general neocortical regions, the DTI-based results put a new focus on attention and flexibility, low-level primary sensory processing, empathy, emotion, and depth of processing. Psychological trait characterization may benefit from diffusion tensor imaging methodology by identifying influential brain systems for the trait.
Methods
Participants
We used data from the Young Adult Human Connectome Project (YA-HCP) WU-Minn-Oxford consortium S500 release (Van Essen et al. 2012; Glasser et al. 2013), from which we used data of 408 subjects (243 females and 165 males) and self-report questionnaire data. Age of the subjects was between 22 and 36 years (mean age for females 29.2 years, standard deviation 3.4 years; mean age for males 28.9 years, standard deviation 3.6 years); ethnicity: 66.18% White/European ancestry, 20.59% African-American, 7.84% Latino, 1.96% Asian or Nat. Hawaiian or other Pacific, 1.96% not reported, 1.47% more than one. The participants were free of documented psychiatric or neurological disorders.
Assessment of sensory processing sensitivity
The YA-HCP does not include the standard HSP scale for SPS, but does include multiple psychological measures. Therefore, we systematically identified and tested a subset of items in the HCP dataset that could serve as a proxy measure. First, the authors of the standard measure, the Highly Sensitive Person (HSP) Scale (Aron and Aron 1997) examined the various self-report measures in the YA-HCP data, which are based on the NIH Toolbox, and selected 55 candidate items to assess SPS. We administered the 55 candidate items along with the standard HSP Scale (in counterbalanced order) to a sample of 401 mTurk workers (crowd sourcing marketplace for questionnaires; see Amazon Mechanical Turk, mturk.com). Of these 401, 19 failed one or more of four attention checks and 1 gave identical responses (all 1 s or all 7 s) to all the items in three of the main scales. The remaining sample of 381 included 174 women, 206 men, and 1 who did not indicate gender; mean age was 35.88 (SD 11.23); 72% White/European ancestry, 8% African-American, 8% Asian, 7% Latino; 5% other.
We randomly divided the mTurk sample’s data into three groups, with the constraint of equal percentages of each gender in each group: group 1, n = 181, groups 2 and 3, n = 100 each. There were no significant differences in age or ethnicity between subgroups. In group 1, we correlated each candidate item with the standard HSP Scale. Using those results, we explored several different subsets of the 55 items, checking each subset both for overall correlation with the HSP Scale and internal validity, and we identified a potentially optimal subset. Next, we administered this subset to group 2 and made further adjustments, then tested this further adjusted version in group 3 and also tested the reliability of this set of items in the overall HCP sample.
The resulting scale consisted of 17 items (see Table 1). In our mTurk sample of 381, the correlation of this 17-item measure with the standard HSP scale was 0.79; adjusting for reliabilities (0.93 for the HSP Scale, 0.79 for the 17-item proxy) yielded a deattenuated correlation of 0.89. This indicates that the 17-item subset is strongly parallel to the standard HSP Scale, and thus an appropriate measure of SPS. The alpha for these 17 items in the YA-HCP dataset was 0.62, which we considered marginally adequate, especially given that these items were taken from separate, not contiguous, scales in the YA-HCP dataset that measure using diverse response types. By contrast, in the mTurk sample, the items from these scales were all administered close to each other. This only marginally adequate reliability does mildly undermine the strength of analyses, suggesting that some failures to find significant results may be due to the low reliability, although significant results obtained in spite of this are likely to be especially robust and may underestimate the actual effect size.
Table 1.
The HSP-Proxy Scale (17 items) developed from questions in the NIH toolbox used for the human connectome project
| From the NIH Toolbox Loneliness (Ages 18 +) – Fixed Form |
| In the past month how often (from 1 = never to 5 = Always) |
| 1. Act like my problems aren’t that important [Soc 276]a |
| 2. Criticize the way I do things [Soc264] |
| From the NIH Toolbox Emotion Anger-Affect (Ages 18 +) – Fixed Form |
| In the past 7 days (from 1 = never to 5 = Always) |
| 3. I was irritated more than people knew [Anger31] |
| 4. I felt annoyed [Anger50] |
| From the NIH Toolbox Fear (Ages 18 +) – Item Bank |
| In the past 7 days (from 1 = never to 5 = Always) |
| 5. I felt uneasy [Anxiety62] |
| From the NIH Toolbox Self-Efficacy (Ages 18 +) – Item Bank/Fixed Form |
| How true is it of you in general (from 1 = never to 5 = Very Often) |
| 6. If I am in trouble, I can think of a solution [GSE09] |
| From the NIH Toolbox Perceived stress (Ages 18 +) – Item Bank/Fixed Form |
| In the past month… (from 1 = never to 5 = Very Often) |
| 7. How often have you been upset because of something that happened unexpectedly? [SC001] |
| 8. How often have you felt nervous and “stressed’’? [SC003] |
| From the NEO-FFI-R (McCrae and Costa 2004), included in the NIH Toolbox |
| Circle the response which best describes your opinion of yourself (from SD Strongly disagree—definitely false to SA Strongly agree—definitely true; for analyses, coded 1 to 5) |
| 9. I am not a worrier [NEORAW__01_Num, reverse coded] |
| 10. I enjoy concentrating on a fantasy or daydream and exploring all its possibilities, letting it grow and develop [NEORAW__03_Num] |
| 11. When I’m under lots of stress, I sometimes feel like I’m going to pieces[NEORAW_11_Num] |
| 12. I am intrigued by the patterns I find in art and nature [NEORAW_13_Num] |
| 13. I often feel tense and jittery [NEORAW_21_Num] |
| 14. I often get angry at the way people treat me [NEORAW_36_Num] |
| 15. I experience a wide range of emotions or feelings [NEORAW_38_Num] |
| 16. When I read a poem or view art, I sometimes feel a wave of excitement[NEORAW_43_Num] |
| 17. Poetry has little or no effect on me [NEORAW__23_Num, reverse coded] |
aCodes in brackets are codes used for items in NIH tool box data sets
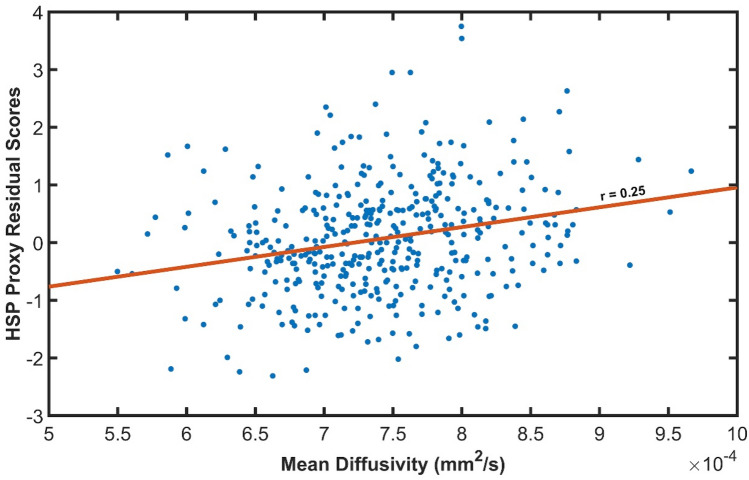
Finally, to control for negative affect, we further adjusted HSP scores. Typically, there is a substantial correlation between HSP scale scores and negative affectivity or neuroticism. Thus, it is standard practice to partial out scores on a measure of negative affect in SPS studies. We did so in the present study by creating standardized residuals of the proxy HSP Scale using mean NEO Neuroticism Scale scores from the sample. Thus, we computed neuroticism-score-adjusted studentized residuals, using a standard model in the statistical program SPSS, which shows HSP scores in terms of a standard deviation having negative and positive values. These scores were used to analyze the data and examples are shown in the graph in Fig. 1.
Fig. 1.
Mean diffusivity correlation with HSP residual score (standard deviations from the mean) in a voxel in the right medial prefrontal cortex, within the cingulum bundle (MNI: 12, 44, −10; see Fig. 2). This voxel showed the highest correlation coefficient from the whole brain analysis (0.247)
Imaging data and processing
We used the minimally processed diffusion magnetic resonance images (dMRI) (Sotiropoulos et al. 2013), from which we selected 90 diffusion-weighted images (DWIs) and 9 non-DWIs available for each participant with b value of 1000 s/mm2 as these images are the most appropriate for a DTI model (Veraart and Sijbers 2016). The dataset has been corrected for subject motion and eddy current-induced distortions via FSL (Jenkinson et al. 2012) tool eddy (Andersson et al. 2003; Andersson and Sotiropoulos 2015) as explained in (Glasser et al. 2013). ExploreDTI version 4.8.6. (Leemans et al. 2009) and the REKINDLE (Tax et al. 2015) tensor estimation approach was used to calculate the voxelwise eigenvalues and eigenvectors. FA, MD, AD, and RD maps were calculated from the fitted tensor model and warped to the MNI template. The native-to-MNI space nonlinear transformation (Fonov et al. 2011) files have been precalculated by the HCP team, which ensured anatomical fidelity to perform voxelwise comparisons. We also corrected for the gradient nonlinearities in the diffusion-weighted gradients (Bammer et al. 2003; Mesri et al. 2019), using the voxelwise pattern of b values and gradient direction during tensor estimation. The mean FA mask was calculated using all subjects and thresholded at FA > 0.2 to identify a white matter mask to limit the spatial extent of the statistical tests. Computations were performed on a Dell multi-core parallel processing system with 72 Intel Xeon E7-8870 v3 at 2.10 GHz dual cores with 1 TB RAM.
Statistical tests
To investigate the correlation between the SPS scores and diffusion measures (FA, MD, AD, and RD), we used the nonparametric t test via Permutation Analysis of Linear Models (PALM) (Holmes et al. 1996; Nichols and Holmes 2003; Winkler et al. 2014; Eklund et al. 2016) with 10,000 iterations. Significance was determined at pcorr < 0.05 using family-wise error rate (FWER) adjustment to correct for multiple comparisons, which corrected for multiple contrasts and modalities, as well (Winkler et al. 2016b). Multiple contrasts are the positive and negative correlations, while multiple modalities are the four inputs, therefore a total of eight tests. Threshold-Free Cluster Enhancement (TFCE) (Smith and Nichols 2009) was used to amplify p values. Calculation speed was accelerated using the tail approximation (Winkler et al. 2016a). Because a large number of subjects can produce highly statistically significant results for small effects, correlation coefficient (r) effect sizes were calculated and are the statistic we emphasize. FWER-corrected p-value maps were fed into the FSL tool automated atlas query (autoaq) to facilitate the anatomical interpretation of the statistically significant voxels. Furthermore, we repeated the above tests including the subjects’ age and sex as confounders as these factors may shadow the effect of interest (Cox et al. 2016; Lawrence et al. 2021).
In a separate analysis, the voxelwise effect size maps were thresholded at r > 0.1, regardless of the associated p values, and the largest connected component (cluster) was selected using the bwconncomp MATLAB function. Exploring the non-trivial effect sizes may provide additional information regarding the spatial extent of the HSP scale—DTI metric relationship.
Region of interest analysis
In addition, we performed region of interest (ROI) analysis for primary sensory processing areas and areas associated with emotion processing based on previous data (Aron et al. 2010; Jagiellowicz et al. 2011; Acevedo et al. 2014, 2017). White matter ROIs were defined by the FreeSurfer (FS) ‘wmparc’ atlas. Thus, the ROIs used larger areas than those detected in previous fMRI studies, which may dilute any smaller regional significant effect. For example, previous studies found functional activations in the angular gyrus, temporoparietal junction, and supramarginal gyrus that are all within the “inferior parietal” ROI region. Thus, we report the findings for some potentially diluted ROIs with p values > 0.05, because these were planned comparisons and hypothesis-driven. The ROIs tested were white matter right and left: bankssts, caudal anterior cingulate, cuneus, entorhinal, fusiform, inferior parietal, inferior temporal, lateral occipital, lateral orbitofrontal, middle temporal, paracentral, parsopercularis, pericalcarine, post-central, precuneus, superior parietal, transverse temporal (primary auditory), and insula.
ROI-based statistical testing was performed similarly to the voxelwise tests for all regional mean DTI metrics. PALM was utilized along with 10,000 iterations with FWER adjustment and tail approximation. Furthermore, regional volume has been demonstrated to influence DTI estimates (Vos et al. 2011). Therefore, the ROI volume was considered as a co-variate of no-interest.
Results
Range of HSP scores and diffusivity values
The raw HSP-proxy questionnaire scores ranged from 1.71 to 3.82 (1–5, possible scores). HSP-proxy residual scores, which are standard deviations from the mean, were used for the analyses and they ranged from − 2.31 to + 3.75 at the maximum effect size voxel (see Fig. 1). The score range allowed an adequate sampling of HSP/SPS trait intensities. Based on approximate cutoffs from (Lionetti et al. 2018) latent class analysis of a large sample using the standard HSP scale, and adjusting their cutoff for mean and SD in the Proxy scale, a score of 2.97 or greater was considered to reflect the HSP trait as influential in everyday life. Sixteen percent (n = 65) of our participants showed this range of scores, a population prevalence estimated by other studies of HSP. Thus, a relatively small but statistically adequate number of our participants would be considered a highly sensitive person, leading to small effect sizes. Mean diffusivity ranged from 5 to 10 × 10–4 mm2/s, values found in normal, healthy brains (Lebel et al. 2008).
HSP-proxy scores correlated with brain axon microarchitecture measures
Whole-brain, exploratory analysis
We found positive correlations between HSP-proxy scores and MD within the ventromedial cingulate, ventromedial, and ventrolateral prefrontal cortex. The largest effects were in the right anterior/ventral subcallosal cingulum bundle, extending into the forceps minor of the corpus callosum (peak r = 0.248, p = 0.017). Figure 1 shows the MD–HSP relationship at the peak effect size (MNI coordinates X: 12, Y: 44, Z: − 10). Other MD effects were in the left anterior–ventral subcallosal cingulum bundle (r = 0.232, p = 0.024). Figure 2A visualizes the voxelwise results from all comparisons on the brain template, while Table 2 lists the regions, MNI coordinates, p values, and effect sizes.
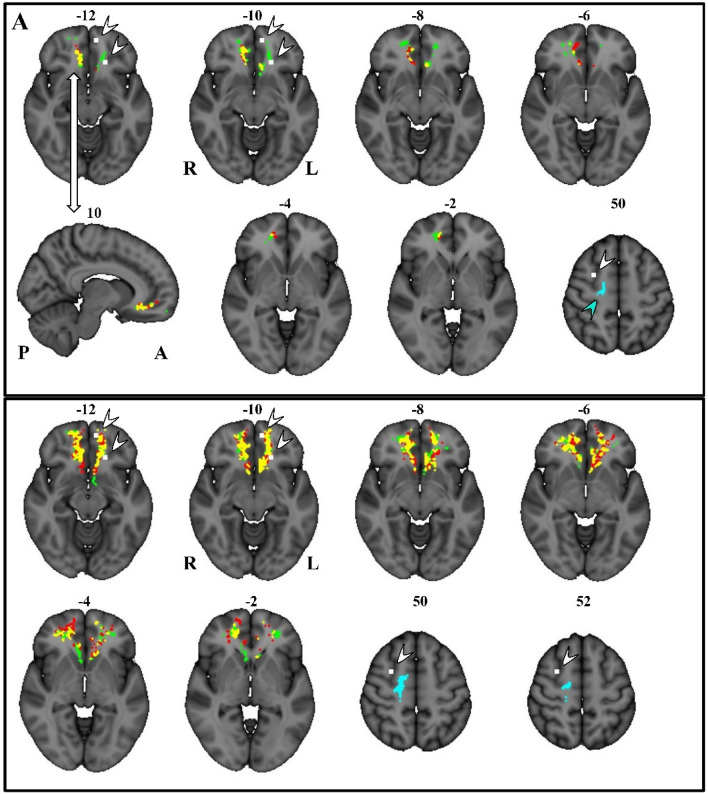
Fig. 2.
Voxelwise results presented in MNI stereotaxic space on axial and sagittal sections. A Voxels in color show statistically significant correlations with HSP-proxy questionnaire scores. They are located in the ventromedial prefrontal cortex, mostly on the right, within the forceps minor of the corpus callosum and the subcallosal cingulum bundle. The correlated voxels extend about 10 mm axially and about 25 mm antero-posteriorly. Significant voxels are also on the left, mostly in the subcallosal area. B Colors show largest voxel clusters above r size of 0.10 without considering statistical significance (see “Methods”). Red: positive correlation with MD (mean diffusivity); green: positive correlation with RD (radial diffusivity); yellow: the overlap of MD and RD; blue and blue arrow: negative correlation with FA (fractional anisotropy); white voxels and white arrows: locations of fMRI activations that correlated with HSP questionnaire scores in a previous study when participants viewed a romantic partner and a stranger, happy, or sad (Acevedo et al. 2014).
Table 2.
Whole-brain exploratory analysis
| Brain region | x | y | z | # of voxels | p-value | Peak r |
|---|---|---|---|---|---|---|
| MD, positive correlation: ventromedial prefrontal cortex/cingulate cortex: | ||||||
| Anterior/ventral subcallosal cingulum bundle and forceps minor of corpus callosum | 12 | 44 | − 10 | 80 | 0.017 | 0.248 |
| Anterior/ventral cingulum bundle | − 6 | 26 | − 10 | 11 | 0.024 | 0.232 |
| RD, positive correlation: ventromedial and lateral prefrontal cortex | ||||||
| Anterior/ventral subcallosal cingulum bundle and forceps minor of corpus callosum | 14 | 42 | − 10 | 57 | 0.022 | 0.214 |
| Anterior/ventral cingulum bundle | 18 | 48 | 0 | 42 | 0.024 | 0.221 |
| Anterior/ventral cingulum bundle | − 6 | 26 | − 10 | 21 | 0.024 | 0.214 |
| FA, negative correlation; motor/premotor cortical region | ||||||
| Premotor area | 20 | − 14 | 52 | 96 | 0.013 | − 0.176 |
| Corticospinal tract | 26 | − 32 | 34 | 29 | 0.021 | − 0.197 |
Brain white matter areas showed positive and negative correlations between diffusion tensor imaging measures and proxy HSP scores for sensory processing sensitivity. X/Y/Z denotes the MNI coordinates for the voxel with peak correlation coefficient within the cluster
There was also a positive correlation for RD in regions overlapping the MD effects in the right anterior/ventral cingulum bundle and forceps minor of the corpus callosum (r = 0.220, p = 0.214) and in a second region of the cingulum bundle (left r = 0.214, p = 0.024; right r = 0.221, p = 0.024; Table 2; Fig. 2A).
Connected voxels showed a continuous band in the ventral cingulate and ventromedial prefrontal white matter from the posterior genu of the corpus callosum and radiation of the straight gyrus to the frontal pole, including the superior longitudinal fasciculus, inferior fronto-occipital fasciculus, uncinate, and arcuate fasciculus, as shown in Fig. 2B. Furthermore, these results extended into the ventrolateral prefrontal cortex. White matter anatomical findings were near gray matter functional activations found previously in SPS subjects reacting to emotional stimuli (Acevedo et al. 2017) and to a romantic partner’s emotional facial expression as shown in (Acevedo et al. 2014).
We also found a negative correlation between HSP-proxy scores and FA (r = − 0.176; p = 0.013) in the right premotor cortex area in the region of the origin of the corticospinal tract (Fig. 2A). Connected voxels covered a large area that included white matter in the region of the dorsomedial prefrontal cortex, premotor cortex, precentral gyrus (motor cortex), post-central gyrus (somatosensory cortex), and supramarginal gyrus (somatosensory association cortex) in the parietal lobe (Figs. 2B, 3). Figure 3 shows the 3D render of the FA cluster, after thresholding only for effect size. This cluster was near gray matter functional activations observed previously in SPS subjects reacting to a romantic partner’s emotional facial expression as shown in Acevedo et al. (2014) (Fig. 2A, B).
Fig. 3.
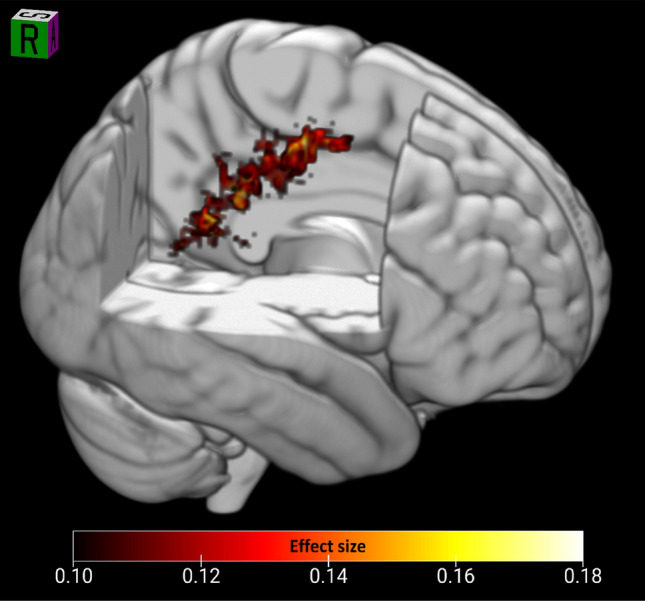
3D render of the largest FA cluster (negatively correlated with HSP score). The cluster extends widely from the premotor cortex through the primary motor and somatosensory cortex into the supramarginal gyrus
Region of interest analysis
Region of interest analyses showed statistically significant positive RD and negative FA correlations with neuroticism-adjusted HSP-proxy scores in two areas. Figures 4 and 5 show the ROI analysis results, while Table 3 shows the numerical summary. RD was positively correlated with scores in the left (r = 0.148, p = 0.030; Fig. 4C) and on the right precuneus (r = 0.144, p = 0.038, Fig. 4B, C). RD showed positive correlations in the right transverse temporal/primary auditory cortex (r = 0.139, p = 0.047; Fig. 4A). FA was negatively correlated with scores in the right parsopercularis/inferior frontal gyrus (r = − 0.165, p = 0.012). Moreover, FA was negatively correlated with scores in the right bank of the superior temporal gyrus (r = − 0.163, p = 0.014; see Table 3; Fig. 4A).
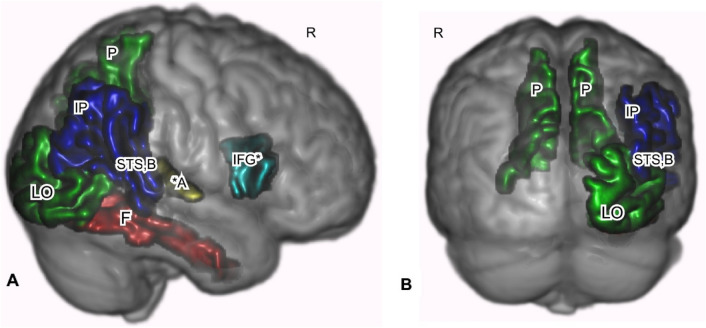
Fig. 4.
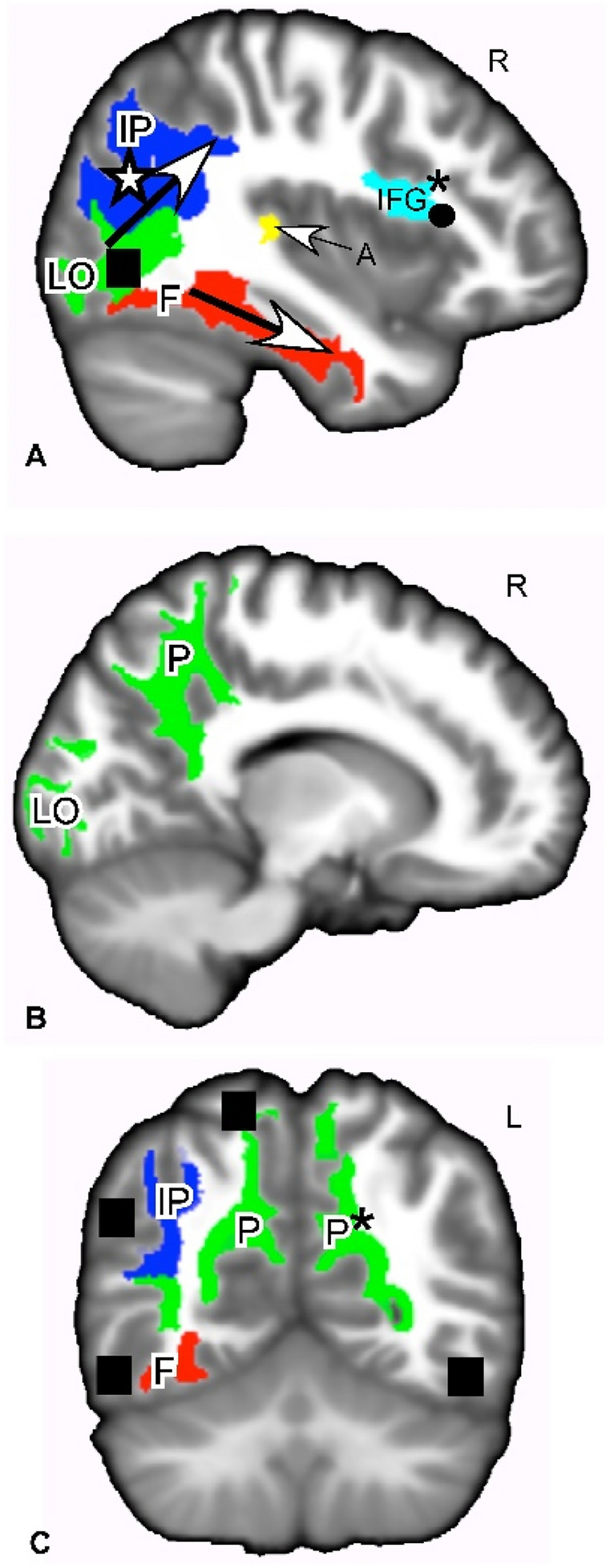
White matter regions of interest where MD, RD, or FA showed positive or negative correlations with HSP-proxy questionnaire scores. A Sagittal slice through ROIs that showed a correlation between HSP-proxy scores and DTI measures. These ROIs are white matter of the ventral pathway for visual processing (LO, F bottom arrow), dorsal pathway for visual processing (IP, top arrow), primary auditory processing (A), and empathic responses (IFG). B Sagittal slice through regions that showed a positive correlation between RD and the HSP-proxy scores. The ROIs are white matter of the ventral and dorsal visual pathways (LO, P). C Coronal slice through white matter regions involved in the ventral and dorsal visual pathway (F, IP, P) that showed correlations between questionnaire scores and MD, RD, and FA. These regions are connected to gray matter areas associated with SPS (filled square). Red: MD positive correlation; green: RD positive correlation; yellow: MD + RD positive correlations; dark blue; FA negative correlation; light blue: FA negative + RD positive correlation. *p < 0.05. Otherwise, p values ranged from 0.06–0.09. Effect sizes − 0.165 to + 0.151. See Table 3. Shape symbols indicate where previous fMRI and behavioral studies of SPS found activations implicating heightened sensory processing, empathy, attention, and self-other processing. Filled square Jagiellowicz et al. (2011), asterisk Acevedo et al. (2014, 2017), filled circle Acevedo et al. (2014). A primary auditory cortex, F fusiform, IFG inferior frontal gyrus, IP inferior parietal/angular gyrus/temporoparietal junction/supramarginal gyrus, LO lateral occipital area, P precuneus, MD mean diffusivity, RD radial diffusivity, FA fractional anisotropy
Table 3.
Region of interest analysis
| White matter regions | Side | MD | RD | FA | |||
|---|---|---|---|---|---|---|---|
| Effect size | P value | Effect size | P value | Effect size | P value | ||
| Inferior frontal gyrus/parsopercularis | R | 0.137 | 0.053 | − 0.165 | 0.012 | ||
| L | − 0.108 | 0.220 | |||||
| Precuneus | R | 0.111 | 0.176 | 0.144 | 0.038 | − 0.124 | 0.117 |
| L | 0.123 | 0.107 | 0.148 | 0.030 | − 0.104 | 0.261 | |
| Superior temporal sulcus, bank | R | 0.119 | 0.12 | − 0.163 | 0.014 | ||
| L | 0.120 | 0.121 | 0.130 | 0.073 | |||
| Transverse temporal/auditory | R | 0.132 | 0.072 | 0.139 | 0.047 | ||
| L | |||||||
| Lateral occipital | R | 0.119 | 0.127 | 0.132 | 0.067 | − 0.109 | 0.214 |
| L | 0.107 | 0.201 | 0.109 | 0.183 | |||
| Fusiform | R | 0.129 | 0.081 | 0.117 | 0.130 | ||
| L | |||||||
| Inferior parietal (angular gyrus, temporoparietal junction, supramarginal gyrus) | R | 0.109 | 0.185 | − 0.139 | 0.057 | ||
| L | |||||||
White matter regions with r effect sizes > 0.1 or statistically significant p values
We report other areas at p > 0.05, because they are primary visual sensory or higher order sensory processing areas that were activated in previous fMRI studies: the primary visual cortex (right lateral occipital area, RD; r = 0.132, p = 0.067, see Table 3; Fig. 4A, B), the right fusiform gyrus (MD; r = 0.129, p = 0.081, see Table 3; Fig. 4A, C), and the inferior parietal cortex that includes the angular gyrus, temporoparietal junction, and supramarginal gyrus (FA; r = − 0.139, p = 0.057; Fig. 4A). These areas (Fig. 4A) are part of the “ventral visual stream” that identifies “what” in the visual field and the “dorsal visual stream” that identifies “where” in the visual field (Ungerleider and Haxby 1994; Milner and Goodale 2008). Functional activations of these regions were seen in SPS individuals during a visual discrimination task (Aron et al. 2010; Jagiellowicz et al. 2011; Acevedo et al. 2012, 2017). Finally, MD was positively correlated with HSP-proxy scores in the right transverse temporal/primary auditory cortex (r = 0.132, p = 0.072; Fig. 4A).
Discussion
Overview
This study established an anatomical correlate in the white matter of the brain for SPS individuals: those with the highest neuroticism-adjusted HSP-proxy scores showed the greatest RD, FA, and MD effects in several neocortical areas compared to those with the lowest HSP scores (not highly sensitive). The interpretation of the direction of the effects, negative or positive correlations, must be limited only to identifying a locus of change (see Microstructural differences: the physiological impact of positive MD/RA and negative FA correlations). Whole-brain, exploratory effects were greatest in brain regions involved in (a) higher order emotion and reward processing: the ventromedial prefrontal cortex; and (b) in regions involved in empathy, self-other processing, attention and flexible coding of the environment: the premotor cortex and supramarginal gyrus. Compared to whole brain results, smaller ROI effects were also seen in self-other processing areas (IFG, precuneus, fusiform, angular gyrus), higher order visual processing regions of the ventral and dorsal pathways (precuneus, inferior parietal, temporoparietal junction, STSb), and primary sensory processing areas, such as the lateral occipital and transverse temporal (primary) auditory cortex.
All of these results are consistent with behavioral observations for SPS, which include sensory sensitivity, a tendency to be overwhelmed by sensory stimuli, and more attention to emotional and visual details of stimuli than others who do not show these traits (Aron and Aron 1997; Aron et al. 2012). The localization of the higher order visual processing effects is also consistent with the previous studies, as reviewed in the Introduction, that used functional MRI to assess details of visual scenes and emotional reactions in highly sensitive people (Jagiellowicz et al. 2011; Acevedo et al. 2014, 2017). Thus, regional functional effects previously described were confirmed by microstructural effects. In addition, this is the first study to suggest involvement of primary sensory processing areas in the cortex, such as the visual and auditory cortex. The study also highlights the premotor cortex, with its connections to the supramarginal gyrus and attention functions. Somatosensory as well as environmental stimuli like vision are new possible highlights. Furthermore, the study suggests a novel focus on the functions of the ventromedial prefrontal cortex. Finally, this is one of few studies to show small DTI anatomical effects within axonal tracts for normal-range behavioral traits, in this case sensory processing. Other studies have investigated neurologically normal subjects for DTI effects, mostly using the “big five” personality traits (Xu and Potenza 2012), but these traits overlap very little with the HSP Scale, other than with neuroticism (which is controlled for in this study). In this study of a normal trait, the results indicate novel behavior-related brain regions to explore in future studies.
Ventromedial prefrontal cortex
The largest effects were in white matter of the ventromedial and ventrolateral prefrontal cortex (De La Vega et al. 2016), on both right and left sides, but with more voxels on the right. These tracts connect major limbic system components: the hippocampus/peri-hippocampal cortex and amygdala to the medial prefrontal cortex. The subcallosal cingulum area, functionally connected with the ventromedial and ventrolateral prefrontal cortex (Dunlop et al. 2017), is known for its influence on mood, especially depression (Mayberg et al. 2013; Dunlop et al. 2017). The cingulum is also structurally connected to several of the ROIs that were correlated with HSP-proxy scores in this study: the precuneus, bank of the superior temporal sulcus, and supramarginal gyrus (Bathelt et al. 2019).
The ventromedial and lateral prefrontal cortex areas are described by Hiser and Koenigs (2018) as having three broad domains of psychological function: decision-making based on reward and value (Sescousse et al. 2013); generation and regulation of negative emotions; and, social cognition, such as facial recognition, theory of mind, and processing self-relevant information. These of course interact strongly, especially given the social nature of humans, as in the function of the vmPFC in making moral decisions (Cameron et al. 2018) or in the “intuitive feeling of rightness” that guides decision-making, often social in nature, as a function of memory retrieval (Hebscher and Gilboa 2016).
The major results in the vmPFC in this study, as well as in the preceding fMRI studies (Acevedo et al. 2014, 2017), point to perhaps the most important aspect of SPS, which is “depth of processing” (Aron et al. 2012). The term is based on cognitive conceptualizations of levels or processing, with the idea that processing to deeper levels with more detailed cognitive contexts leads to better memory and better learning overall (Lockhart et al. 1976; Leow 2018). The hypothesized evolutionary development of depth of processing in SPS is based on a computer simulation demonstrating that unusual responsivity to the environment will evolve when there are enough payoffs for an individual difference in noticing details, as long as most individuals do not notice these details (Wolf et al. 2008). (If all individuals did, there would be no special benefit.) That is, SPS is considered fundamentally an individual difference in “depth of processing” through careful observation of situation/time A to compare those details in memory to situation/time B and gain any potential benefits others miss. Individuals without the trait are thought not to process A as carefully, a strategy which is often equally or more effective, since B may bear no resemblance to A or there may be little reward in noticing any resemblances.
This type of careful processing relies on emotional motivation, the desire for rewards, such as winning, and the desire to avoid fear-related stimuli, such as losing (Baumeister et al. 2007). Hence, understanding SPS as fundamentally about depth of processing for decision-making based on reward value and social value is consistent with the significant differences that were found in the vmPFC, with its close connections to emotion-related memory processing areas such as the hippocampus and amygdala. Memory enhanced by motivation is, again, key to SPS, and specific activations occur for those high in SPS when processing emotional stimuli. For example, Acevedo et al. (2017) found in their comparison of responses to positive and negative stimuli that there were considerable differences in regional brain activation for high and low SPS in the vmPFC, in the same areas where microstructural differences were found in this study (Fig. 2), and in regions that mediate memory, attention, awareness, and reflective thinking.
Theory of Mind
In a meta-analytic review of the Theory of Mind, Mar (2011) highlighted a core mentalizing network: the mPFC, precuneus, bilateral pSTS, bilateral angular gyri, and the right IFG. These structures showed microstructural differences associated with SPS in this study, notably the mPFC, right IFG, precuneus, the bank of the STS, and angular gyrus region. Second, this mentalizing network overlaps with the narrative comprehension network in a number of areas, including the mPFC, bilateral pSTS/TPJ, precuneus, and possibly the right IFG, again areas implicated by this study. Theory of Mind is central to the core concept of SPS, in that this survival strategy would require more reflection on another’s behavior to accurately see from their perspective and correctly attribute to them their motivations and intentions.
Premotor cortex, attention, and somatosensory processing
Finally, regarding the extensive cluster from the left premotor cortex to the post-central somatosensory and supramarginal gyrus, it could be expected that the arc of axonal effects we found in sensory processing areas from posterior to anterior (Figs. 2B, 4A, 5A) would include the premotor cortex, where the hypothesized deep processing associated with SPS would result in the preparation for action. This potential somatosensory/posterior parietal/premotor cortex involvement in SPS is a novel contribution to its understanding and may be helpful in future studies.
Fig. 5.
Primary sensory, higher order sensory processing, and cognitive processing regions were identified as correlated with the HSP-proxy scores. Freesurfer-based ROIs are shown for white matter where correlation coefficient effect size was − 0.165 to 0.148, rendered in 3D on a template brain. Areas include the general path of the dorsal and ventral visual pathways. A Sagittal view of the right side. B Posterior view. Red: MD positive correlation with HSP-proxy questionnaire scores. Green: RD positive correlation. Blue: FA negative correlation. Gold: MD and RD positive correlation. Aquamarine in IFG: RD and FA correlations. *p < 0.05. Otherwise, p values were 0.06–0.09. See Table 3. A primary auditory cortex, F fusiform, IFG inferior frontal gyrus, IP inferior parietal including angular gyrus/temporoparietal junction/supramarginal gyrus, LO lateral occipital cortex, P precuneus, STS B-bank of the superior temporal sulcus, MD mean diffusivity, RD radial diffusivity, FA fractional anisotropy
The importance of this broad premotor area continues to evolve (Rizzolatti et al. 1987). Rizzolatti et al. suggested a theory of attention focused on this area, presenting evidence that the premotor areas were the source of attention rather than a separate attention-directing mechanism. That is, attention is turned to a stimulus within the premotor area, so that attention consists of nothing more than preparation for a motor activity (e.g., an eye movement toward a stimulus deemed important or auditory areas preparing for a sound). Although refinements and extensions (Wollenberg et al. 2018) have occurred, this view of attention is still tenable, according to experiments by (Schubotz and Von Cramon 2003).
Schubotz and Cramon distinguished the left premotor cortex, correlated in our study with SPS, as associated with nonspatial tasks and rapid acquisition of new motor sequences. Overall, evidence regarding the premotor area suggests “environmental features do not have to remind us of specific actions or movements to induce premotor activation on a more or less conscious level. Rather, features are represented in a highly fragmented format that allows for instant recombination and very flexible coding of any currently attended environment” (p. 126). Schubotz (2007) presented considerable evidence that the premotor area (along with most of the areas in the brain associated with SPS) helps in the prediction of events.
Furthermore, a mean diffusivity study by Takeuchi et al. (2019) found that an area including the premotor cortex plays a major role in emotional salience and empathy. This area was also activated in the (Acevedo et al. 2014) fMRI study finding empathy for happy and distressed partners and strangers.
Attention, flexible coding, prediction, somatosensory processing, and empathy all fit with the theory that SPS involves attending to subtle stimuli that may be relevant for survival and predicting those environmental details that what will be relevant in future environments. Overall, these whole brain, exploratory analyses provide a picture that is consistent with behavior associated with SPS.
ROI results
The particular ROIs with clear statistical significance were the left precuneus (RD, r = 0.148, p = 0.030), part of the dorsal visual stream, and the right parsopercularis/IFG (FA, r = − 0.165, p = 0.012), associated with empathy. For the precuneus, the other side was significant too (RD, r = 0.144, p = 0.038), while for the IFG, the same side was marginally significant for RD, as well (r = 0.137, p = 0.053). Also, the transverse temporal gyrus/primary auditory cortex (A1), part of the dorsal auditory stream was marginally statistically significant (MD, r = 0.132, p = 0.072).
As for the precuneus, fMRI studies (Cavanna and Trimble 2006) of healthy subjects suggest that it plays a major role in visuo-spatial imagery, episodic memory retrieval, and self-processing tasks, such as the experience of agency and taking the first-person perspective. All of these activities are more prominent in those high in SPS, and the precuneus was another area often correlated with SPS in fMRI studies. There is also a hypothesized role for the precuneus in consciousness itself (Cavanna 2007), along with areas nearby in the posteromedial parietal cortex. It is especially active during the conscious resting state, but is deactivated when consciousness decreases (e.g., sleep, anesthesia, Alzheimer’s disease). Indeed, it has been proposed that it is part of a larger network that correlates with self-consciousness, as it engages in self-related mental representations, self-reflection, and autobiographical memory retrieval. Meditation, which creates states of restful alertness, is associated with microstructural differences in the precuneus in practitioners compared to controls (Shao et al. 2016; Avvenuti et al. 2020). Without suggesting that SPS somehow results in more consciousness, it may well demonstrate an internal tendency for more awareness and integration of diverse aspects of inner and outer experience. Although the insula was not a factor in this study, it has a similar role in the brain and was found to be more active in the fMRI studies of SPS already cited, and has also sometimes been described as the “seat of consciousness” (Craig 2009).
The right parsopercularis/IFG region was negatively associated with HSP-proxy scores and FA (p = 0.02), and positively associated with RD (p = 0.07). IFG functions may be particularly important to recognize in further studies. In an fMRI study, the IFG region was positively associated with HSP scores during positive emotion conditions while looking at a spouse or stranger (Acevedo et al. 2014). It has been identified with a mirror neuron system (Iacoboni et al. 1999; Jabbi and Keysers 2008; Van Overwalle and Baetens 2009) that responds to the movements of others, and may facilitate the understanding of others’ intentions and feeling of empathy. We previously suggested that this system’s activation is consistent with HSPs’ bias toward noticing positive expressions in others and high empathy (Acevedo et al. 2014).
Some of the ROI effects are along the dorsal and ventral visual/auditory pathways, which is especially noteworthy and we speculate that these pathways contribute to depth of processing in SPS. These pathways are described as identifying the “what” (ventral stream) and “where” (dorsal stream) of what is seen and heard, taking sensory experience beyond its initial input (Milner and Goodale 2008). The ventral stream in particular is associated with object recognition and form representation, the “what,” and is strongly connected to the medial temporal lobe, which stores long-term memories; the limbic system, which controls emotions; and joins with the dorsal stream, which identifies the “where.” The dorsal stream is said to guide actions and recognize where objects are in space. It stretches from the primary visual cortex in the occipital lobe into the parietal lobe. It contains a detailed map of the visual field and serves to detect and analyze movements. Thus, it commences with purely visual functions, ending with spatial awareness at its termination. As with the ventral stream, processing of sensory input along the dorsal stream becomes “deeper” or more elaborate. It ends up contributing to recognizing spatial relations, body image, and physical coordination. Again, as SPS has been described, the trait is not characterized by better initial sensory perception, better hearing or eyesight, but by more complete processing of what is perceived along these two visual/auditory pathways. However, the potential involvement of primary visual, auditory, and somatosensory areas suggested in this study leads to other questions for study of the most basic sensory detection and discrimination functions of these areas that may impact the higher order processing regions.
The A1 cortex that we included as an ROI for this study is thought to operate very early in the recognition of sounds. For example, a study by Warrier et al. (2009) found that non-Mandarin-speaking subjects who could successfully form an association between Mandarin Chinese “pitch patterns” and word meaning were found to have transverse temporal gyri (A1) with larger volume than subjects who had difficulty learning these associations. Successful completion of the task also was associated with a greater concentration of white matter in the left A1 of the subject. In general, larger transverse temporal gyri seemed to be associated with more efficient processing of speech-related cues, which could aid the learning and perceiving of new speech sounds. The A1 cortex is also associated with inner speech, what Hurlburt et al. (2016) might be considered a more advanced level of processing, but still preceding speech production. Although, to date, there are no studies of auditory functioning associated with SPS, it would seem to be a fruitful area for future research.
The superior temporal sulcus (STS bank, p = 0.06) is seen primarily as an area for higher visual processing. Hein and Knight (2008), in a review of carefully selected fMRI studies, concluded that the majority of findings implicate the STS in broader tasks involving theory of mind, audiovisual integration, motion processing, speech processing, and face processing. They conclude that rather than trying to pinpoint where in the STS these occur, it is best to view the function of the STS as varying according to the nature of network coactivations with different regions in the frontal cortex and medial temporal lobe during a particular task. This view is more in keeping with the notion that the same brain region can support different cognitive operations depending on task-dependent network connections, emphasizing the important role of network connectivity analysis in neuroimaging. It is consistent with current hypotheses about SPS that those high in SPS would show greater microstructural differences in an area associated with diverse types of processing (motion, speech, face, and audiovisual) as well as theory of mind.
Microstructural differences: the physiological impact of positive MD/RA and negative FA correlations
The physiological impact and thus psychological effects of positive MD/RA and negative FA correlations in normal brain are unclear (Soares et al. 2013). Therefore, the interpretation of negative or positive DTI effects must be limited to identifying only a locus of change in SPS rather than identifying higher or lower speed of processing in SPS. For example, a negative FA correlation in one-fiber system may indicate release of activity in a target area that increases depth of processing. It is different from disease states.
Indeed, the findings in this study are smaller than those seen in previous studies of disease progression or aging (Voineskos et al. 2012; Nir et al. 2013). There is no neurological or behavioral pathology in the group that we studied. The psychological traits measured are subtle and part of the normal range of human behavior. Thus, the effects are part of a normal variability in the population, but may be markers of slight anatomical differences in axon size and organization, perhaps impacting the speed of communication among regions (Horowitz et al. 2015).
Explaining complex human behavioral traits with imaging-derived biomarkers remains challenging (Young et al. 2020), because different microstructural features can contribute to a similar signal profile. Therefore, it is not possible to exclusively attribute a particular processing effect to the observed statistical negative or positive correlations. However, animal studies in traumatic brain injury (TBI) showed reduced FA and increased MD at the impact site due to demyelination and edema (Bigler and Maxwell 2012; Pasternak et al. 2016). Decreased FA can be the result of decreased diffusion hindrance as well as axonal loss (Harsan et al. 2006; Budde et al. 2011), while an increase in RD and unchanged AD was observed during myelin loss alone (Song et al. 2002; Roosendaal et al. 2009; Stricker et al. 2009), which increases MD as MD is the weighted average of AD and RD. Results from the ENIGMA group showed widespread decrease in FA and increases in MD and RD (Young et al. 2020) among people with schizophrenia compared to healthy controls. However, we cannot state that relatively higher HSP score volunteers are affected by edema or loss of myelin at the particular locations, because their MRIs were normal. Again, it is the locus of a change that is important; a decrease in FA does not indicate schizophrenia or any other mental disorder, either because the anatomical distribution of the changes is so different.
This study joins others that have looked at microstructural correlates of normal individual differences, such as personality traits (Xu and Potenza 2012) and cognitive abilities (Bathelt et al. 2019). Since such phenotypic differences can be caused by multiple genetic and environmental effects, looking for common microstructure may be another useful way to identify such differences, even though they may be small.
Limitations
A limitation of the study is its reliance on a proxy measure of SPS. A few questions in the YA-HCP questionnaire dataset addressed sensory processing directly. However, the proxy measure was found in independent samples to have a strong correlation with the standard measure (r = 0.79; r = 0.89 adjusting for reliabilities). Another limitation is the fact that we assessed microstructure properties with only two analysis methods (voxelwise and region-based) using one particular model (DTI). There are a number of additional analysis (David et al. 2021) and dMRI modeling techniques (Tournier et al. 2011; Mori and Tournier 2014) which can assess the current research questions from different perspectives and would be useful in future studies of SPS. Throughout this work, we did not consider a whole family of analysis modes, which utilizes the virtually reconstructed structural connections using the underlying diffusion orientation information, also known as fiber tractography (FT) (Jones 2010). The application of FT opens up a number of new ways to perform statistical comparisons: tract-based (Lebel et al. 2008); along-the-track (Reijmer et al. 2013); connectivity or connectome (Rubinov and Sporns 2010) as well as disconnectome (Thiebaut de Schotten et al. 2020); and tract geometry-based (Yeh 2020) among others, which would be useful in future studies. Also, from a modeling perspective, DTI has the theoretical limitation that it cannot resolve multiple fiber orientations within a voxel. As a result, tractography may provide inadequate results for certain pathways (Jeurissen et al. 2019), while relatively short association fibers are challenging or nearly impossible to map with DTI (David et al. 2019). One of the most notable solutions is called constrained spherical deconvolution (CSD) by Tournier et al. (2007). In CSD, a set of spherical harmonics are calculated to model the fiber orientation distributions (FODs). For tractography-based applications, modeling with CSD and like methods is necessary, since nearly all white matter voxels in the brain contain multiple fiber orientations (Jeurissen et al. 2013). Future aims of research more generally will be to replicate these findings and to examine the relation of SPS to brain structure with DTI and other dMRI-based techniques in younger and older age groups and in other populations.
Conclusions
This is the first study to investigate the relation of SPS to neural anatomical measures using DTI. The study employed a relatively large sample of young healthy individuals, and it has identified several brain systems that may be critical to fully understanding the SPS trait, such as parietal/premotor connections. The study has also confirmed the involvement of several brain systems and areas previously correlated with SPS in fMRI studies, such as those for empathy and higher order visual scene processing. Future research should focus on primary visual and auditory processing; higher order somatosensory processing; attention flexibility and reward value processing. Also, the development of a proxy measure allows future research to examine the relation of SPS to other variables in the large YA-HCP sample, such as various genetic, functional imaging, and self-report data. Finally, DTI may be a valuable and fairly straightforward approach for future psychological and anatomical studies of normal individual differences, because the scan can be acquired quickly and is often included in the usual battery of clinical scans.
Author contributions
SD: conceptualization, methodology, formal analysis, and writing—original draft, visualization; LLB: conceptualization, methodology, writing—original draft, writing—review and editing, and visualization; AMH: conceptualization, and writing—review and editing; EA: conceptualization, writing—original draft, and writing—review and editing; AL: methodology, writing—review and editing, supervision, and funding acquisition; AA: conceptualization, methodology, formal analysis, writing—original draft, writing—review and editing, and supervision.
Funding
The research of S.D. and A.L. is supported by VIDI Grant 639.072.411 from the Netherlands Organization for Scientific Research (NWO).
Data availability
All data are available at https://db.humanconnectome.org.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Szabolcs David, Lucy L. Brown, Alexander Leemans, and Arthur Aron have contributed equally to this work.
References
- Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Soc Cogn Affect Neurosci. 2012;7:145–159. doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo BP, Aron EN, Aron A, et al. The highly sensitive brain: an fMRI study of sensory processing sensitivity and response to others’ emotions. Brain Behav. 2014;4:580–594. doi: 10.1002/brb3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acevedo BP, Jagiellowicz J, Aron E, et al. Sensory processing sensitivity and childhood quality’s effects on neural responses to emotional stimuli. Clin Neuropsychiatry. 2017;14:359–373. [Google Scholar]
- Acevedo B, Aron E, Pospos S, Jessen D. The functional highly sensitive brain: a review of the brain circuits underlying sensory processing sensitivity and seemingly related disorders. Philos Trans R Soc B Biol Sci. 2018 doi: 10.1098/rstb.2017.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. Neuroimage. 2015;122:166–176. doi: 10.1016/j.neuroimage.2015.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- Aron EN, Aron A. Sensory-processing sensitivity and its relation to introversion and emotionality. J Personal Soc Psychol. 1997 doi: 10.1037/0022-3514.73.2.345. [DOI] [PubMed] [Google Scholar]
- Aron EN, Aron A, Davies KM. Adult shyness: the interaction of temperamental sensitivity and an adverse childhood environment. Personal Soc Psychol Bull. 2005;31:181–197. doi: 10.1177/0146167204271419. [DOI] [PubMed] [Google Scholar]
- Aron A, Ketay S, Hedden T, et al. Temperament trait of sensory processing sensitivity moderates cultural differences in neural response. Soc Cogn Affect Neurosci. 2010;5:219–226. doi: 10.1093/scan/nsq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron EN, Aron A, Jagiellowicz J. Sensory processing sensitivity: a review in the light of the evolution of biological responsivity. Personal Soc Psychol Rev. 2012;16:262–282. doi: 10.1177/1088868311434213. [DOI] [PubMed] [Google Scholar]
- Assary E, Zavos HMS, Krapohl E, et al. Genetic architecture of environmental sensitivity reflects multiple heritable components: a twin study with adolescents. Mol Psychiatry. 2020 doi: 10.1038/s41380-020-0783-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avvenuti G, Leo A, Cecchetti L, et al. Reductions in perceived stress following transcendental meditation practice are associated with increased brain regional connectivity at rest. Brain Cogn. 2020;139:105517. doi: 10.1016/j.bandc.2020.105517. [DOI] [PubMed] [Google Scholar]
- Bammer R, Markl M, Barnett A, et al. Analysis and generalized correction of the effect of spatial gradient field distortions in diffusion-weighted imaging. Magn Reson Med. 2003;50:560–569. doi: 10.1002/mrm.10545. [DOI] [PubMed] [Google Scholar]
- Bathelt J, Johnson A, Zhang M, Astle DE. The cingulum as a marker of individual differences in neurocognitive development. Sci Rep. 2019 doi: 10.1038/s41598-019-38894-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD, DeWall CN, Zhang L. How emotion shapes behavior: feedback, anticipation, and reflection, rather than direct causation. Personal Soc Psychol Rev. 2007;11:167–203. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bigler ED, Maxwell WL. Neuropathology of mild traumatic brain injury: relationship to neuroimaging findings. Brain Imaging Behav. 2012;6:108–136. doi: 10.1007/s11682-011-9145-0. [DOI] [PubMed] [Google Scholar]
- Budde MD, Janes L, Gold E, et al. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134:2248–2260. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron CD, Reber J, Spring VL, Tranel D. Damage to the ventromedial prefrontal cortex is associated with impairments in both spontaneous and deliberative moral judgments. Neuropsychologia. 2018;111:261–268. doi: 10.1016/j.neuropsychologia.2018.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE. The precuneus and consciousness. CNS Spectr. 2007;12:545–552. doi: 10.1017/S1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Tucker-Drob EM, et al. Ageing and brain white matter structure in 3,513 UK biobank participants. Nat Commun. 2016;7:13629. doi: 10.1038/ncomms13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- David S, Heemskerk AM, Corrivetti F, et al. The superoanterior fasciculus (SAF): a novel white matter pathway in the human brain? Front Neuroanat. 2019;13:1–18. doi: 10.3389/fnana.2019.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Verhoeff J, Leemans A. Chapter 10—Diffusion MRI analysis methods. In: Choi I-Y, Jezzard P, editors. Advanced neuro MR techniques and applications. Cambridge: Academic Press; 2021. pp. 147–156. [Google Scholar]
- De La Vega A, Chang LJ, Banich MT, et al. Large-scale meta-analysis of human medial frontal cortex reveals tripartite functional organization. J Neurosci. 2016;36:6553–6562. doi: 10.1523/JNEUROSCI.4402-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop K, Hanlon CA, Downar J. Noninvasive brain stimulation treatments for addiction and major depression. Ann N Y Acad Sci. 2017;1394:31–54. doi: 10.1111/nyas.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016;113:7900–7905. doi: 10.1073/pnas.1602413113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, et al. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, et al. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 2013;80:105–124. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greven CU, Lionetti F, Booth C, et al. Sensory processing sensitivity in the context of environmental sensitivity: a critical review and development of research agenda. Neurosci Biobehav Rev. 2019;98:287–305. doi: 10.1016/j.neubiorev.2019.01.009. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Hebscher M, Gilboa A. A boost of confidence: the role of the ventromedial prefrontal cortex in memory, decision-making, and schemas. Neuropsychologia. 2016;90:46–58. doi: 10.1016/j.neuropsychologia.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Hedden T, Ketay S, Aron A, et al. Cultural influences on neural substrates of attentional control. Psychol Sci. 2008;19:12–17. doi: 10.1111/j.1467-9280.2008.02038.x. [DOI] [PubMed] [Google Scholar]
- Hein G, Knight RT. Superior temporal sulcus—it’s my area: or is it? J Cogn Neurosci. 2008;20:2125–2136. doi: 10.1162/jocn.2008.20148. [DOI] [PubMed] [Google Scholar]
- Hiser J, Koenigs M. The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol Psychiatry. 2018;83:638–647. doi: 10.1016/j.biopsych.2017.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JDG, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Horowitz A, Barazany D, Tavor I, et al. In vivo correlation between axon diameter and conduction velocity in the human brain. Brain Struct Funct. 2015;220:1777–1788. doi: 10.1007/s00429-014-0871-0. [DOI] [PubMed] [Google Scholar]
- Hurlburt RT, Alderson-Day B, Kuhn S, Fernyhough C. Exploring the ecological validity of thinking on demand: neural correlates of elicited vs spontaneously occurring inner speech. PLoS ONE. 2016;11:e0147932. doi: 10.1371/journal.pone.0147932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, et al. Cortical mechanisms of human imitation. Science (80-) 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Keysers C. Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion. 2008;8:775–780. doi: 10.1037/a0014194. [DOI] [PubMed] [Google Scholar]
- Jagiellowicz J, Xu X, Aron A, et al. The trait of sensory processing sensitivity and neural responses to changes in visual scenes. Soc Cogn Affect Neurosci. 2011;6:38–47. doi: 10.1093/scan/nsq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, et al. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Jeurissen B, Leemans A, Tournier JD, et al. Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp. 2013;34:2747–2766. doi: 10.1002/hbm.22099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeurissen B, Descoteaux M, Mori S, Leemans A. Diffusion MRI fiber tractography of the brain. NMR Biomed. 2019;32:e3785. doi: 10.1002/nbm.3785. [DOI] [PubMed] [Google Scholar]
- Jones DK. Diffusion MRI: theory, methods, and application. Oxford: Oxford University Press; 2010. [Google Scholar]
- Kagan J. Temperament and the reactions to unfamiliarity. Child Dev. 1997 doi: 10.1111/j.1467-8624.1997.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Karam EG, Fayyad JA, Farhat C, et al. Role of childhood adversities and environmental sensitivity in the development of post-traumatic stress disorder in war-exposed Syrian refugee children and adolescents. Br J Psychiatry. 2019 doi: 10.1192/bjp.2018.272. [DOI] [PubMed] [Google Scholar]
- Lawrence KE, Nabulsi L, Santhalingam V, et al. Age and sex effects on advanced white matter microstructure measures in 15,628 older adults: a UK biobank study. Brain Imaging Behav. 2021;15:2813–2823. doi: 10.1007/s11682-021-00548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, et al. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage. 2008;40:1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Leemans A, Jeurissen B, Sijbers J, et al. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc Int Soc Magn Reson Med. 2009;17:3537. doi: 10.1093/occmed/kqr069. [DOI] [Google Scholar]
- Leow R. Explicit learning and depth of processing in the instructed setting: theory, research, and practice. Stud Engl Educ. 2018;23:769–801. doi: 10.22275/see.23.4.01. [DOI] [Google Scholar]
- Lionetti F, Aron A, Aron EN, et al. Dandelions, tulips and orchids: evidence for the existence of low-sensitive, medium-sensitive and high-sensitive individuals. Transl Psychiatry. 2018;8:1–11. doi: 10.1038/s41398-017-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart RS, Craik FIM, Jacoby L. Depth of processing, recognition and recall. In: Brown J, editor. Recall and recognition. John Wiley & Sons; 1976. [Google Scholar]
- Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti MM, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Depress Sci Ment Health. 2013;6:245–253. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. A contemplated revision of the NEO Five-Factor Inventory. Pers Individ Dif. 2004;36:587–596. doi: 10.1016/S0191-8869(03)00118-1. [DOI] [Google Scholar]
- Mesri HY, David S, Viergever MA, Leemans A. The adverse effect of gradient nonlinearities on diffusion MRI: from voxels to group studies. Neuroimage. 2019 doi: 10.1016/j.neuroimage.2019.116127. [DOI] [PubMed] [Google Scholar]
- Milner AD, Goodale MA. Two visual systems re-viewed. Neuropsychologia. 2008;46:774–785. doi: 10.1016/j.neuropsychologia.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Mori S, Tournier D. Introduction to diffusion tensor imaging. 2. San Diego: Academic Press; 2014. Chapter 8 - Moving beyond DTI: high angular resolution diffusion imaging (HARDI) pp. 65–78. [Google Scholar]
- Nichols T, Holmes A. Nonparametric permutation tests for functional neuroimaging. Hum Brain Funct Second Ed. 2003;25:887–910. doi: 10.1016/B978-012264841-0/50048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir TM, Jahanshad N, Villalon-Reina JE, et al. Effectiveness of regional DTI measures in distinguishing Alzheimer’s disease, MCI, and normal aging. NeuroImage Clin. 2013;3:180–195. doi: 10.1016/j.nicl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini A, Menesini E, Pluess M. The personality trait of environmental sensitivity predicts children’s positive response to school-based antibullying intervention. Clin Psychol Sci. 2018;6:848–859. doi: 10.1177/2167702618782194. [DOI] [Google Scholar]
- Pasternak O, Kubicki M, Shenton ME. In vivo imaging of neuroinflammation in schizophrenia. Schizophr Res. 2016;173:200–212. doi: 10.1016/j.schres.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluess M. Individual differences in environmental sensitivity. Child Dev Perspect. 2015;9:138–143. doi: 10.1111/cdep.12120. [DOI] [Google Scholar]
- Pluess M, Belsky J. Differential susceptibility to parenting and quality child care. Dev Psychol. 2010;46:379–390. doi: 10.1037/a0015203. [DOI] [PubMed] [Google Scholar]
- Pluess M, Boniwell I. Sensory-processing sensitivity predicts treatment response to a school-based depression prevention program: evidence of vantage sensitivity. Pers Individ Difer. 2015;82:40–45. doi: 10.1016/j.paid.2015.03.011. [DOI] [Google Scholar]
- Pluess M, Assary E, Lionetti F, et al. Environmental sensitivity in children: development of the highly sensitive child scale and identification of sensitivity groups. Dev Psychol. 2018 doi: 10.1037/dev0000406. [DOI] [PubMed] [Google Scholar]
- Reijmer YD, Freeze WM, Leemans A, Biessels GJ. The effect of lacunar infarcts on white matter tract integrity. Stroke. 2013;44:2019–2021. doi: 10.1161/STROKEAHA.113.001321. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umiltá C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Geurts JJG, Vrenken H, et al. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009;44:1397–1403. doi: 10.1016/j.neuroimage.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Schubotz RI. Prediction of external events with our motor system: towards a new framework. Trends Cogn Sci. 2007;11:211–218. doi: 10.1016/j.tics.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Schubotz RI, Von Cramon DY (2003) Functional-anatomical concepts of human premotor cortex: evidence from fMRI and PET studies. In: NeuroImage. Academic Press Inc., pp. S120–S131. 10.1016/j.neuroimage.2003.09.014 [DOI] [PubMed]
- Sescousse G, Caldú X, Segura B, Dreher JC. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neurosci Biobehav Rev. 2013;37:681–696. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Shao R, Keuper K, Geng X, Lee TMC. Pons to posterior cingulate functional projections predict affective processing changes in the elderly following eight weeks of meditation training. EBioMedicine. 2016;10:236–248. doi: 10.1016/j.ebiom.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, Sousa N. A hitchhiker’s guide to diffusion tensor imaging. Front Neurosci. 2013;7:1–14. doi: 10.3389/fnins.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos SN, Jbabdi S, Xu J, et al. Advances in diffusion MRI acquisition and processing in the human connectome project. Neuroimage. 2013;80:125–143. doi: 10.1016/j.neuroimage.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NH, Schweinsburg BC, Delano-Wood L, et al. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesis. Neuroimage. 2009;45:10–16. doi: 10.1016/j.neuroimage.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, et al. Empathizing associates with mean diffusivity. Sci Rep. 2019 doi: 10.1038/s41598-019-45106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tax CMW, Otte WM, Viergever MA, et al. REKINDLE: robust extraction of kurtosis indices with linear estimation. Magn Reson Med. 2015;73:794–808. doi: 10.1002/mrm.25165. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Foulon C, Nachev P. Brain disconnections link structural connectivity with function and behaviour. Nat Commun. 2020 doi: 10.1038/s41467-020-18920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier JD, Calamante F, Connelly A. Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage. 2007;35:1459–1472. doi: 10.1016/j.neuroimage.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Tournier J-D, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magn Reson Med. 2011;65:1532–1556. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. “What” and “where” in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, et al. The human connectome project: a data acquisition perspective. Neuroimage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraart J, Sijbers J. Diffusion kurtosis imaging. In: Van Hecke W, Emsell L, Sunaert S, editors. Diffusion tensor imaging: a practical handbook. New York: Springer; 2016. pp. 407–418. [Google Scholar]
- Voineskos AN, Rajji TK, Lobaugh NJ, et al. Age-related decline in white matter tract integrity and cognitive performance: a DTI tractography and structural equation modeling study. Neurobiol Aging. 2012;33:21–34. doi: 10.1016/j.neurobiolaging.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos SB, Jones DK, Viergever MA, Leemans A. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 2011;55:1566–1576. doi: 10.1016/j.neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- Warrier C, Wong P, Penhune V, et al. Relating structure to function: Heschl’s gyrus and acoustic processing. J Neurosci. 2009;29:61–69. doi: 10.1523/JNEUROSCI.3489-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, et al. Permutation inference for the general linear model. Neuroimage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Douaud G, et al. Faster permutation inference in brain imaging. Neuroimage. 2016;141:502–516. doi: 10.1016/j.neuroimage.2016.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Webster MA, Brooks JC, et al. Non-parametric combination and related permutation tests for neuroimaging. Hum Brain Mapp. 2016;37:1486–1511. doi: 10.1002/hbm.23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Van Doorn GS, Weissing FJ. Evolutionary emergence of responsive and unresponsive personalities. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0805473105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenberg L, Deubel H, Szinte M. Visual attention is not deployed at the endpoint of averaging saccades. PLoS Biol. 2018;16:e2006548. doi: 10.1371/journal.pbio.2006548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Potenza MN. White matter integrity and five-factor personality measures in healthy adults. Neuroimage. 2012;59:800–807. doi: 10.1016/j.neuroimage.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC. Shape analysis of the human association pathways. Neuroimage. 2020;223:117329. doi: 10.1016/j.neuroimage.2020.117329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PNE, Estarellas M, Coomans E, et al. Imaging biomarkers in neurodegeneration: current and future practices. Alzheimers Res Ther. 2020;12:49. doi: 10.1186/s13195-020-00612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available at https://db.humanconnectome.org.