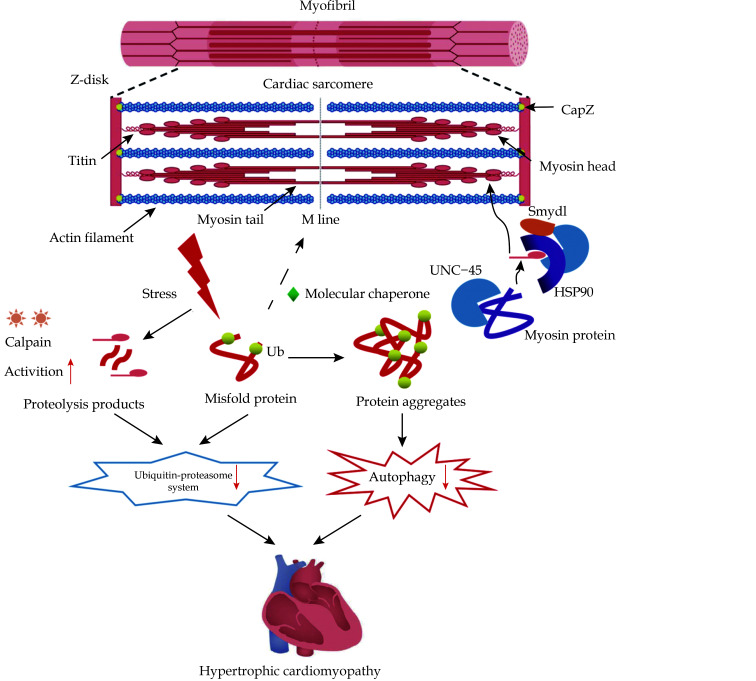
Figure 2.
Schematic diagram of action mechanism of sarcomere protein quality control in hypertrophic cardiomyopathy.
BAG3/HSC70 chaperone complex maintains CapZ stability in sarcomeres. UNC-45 coordinates the folding of myosin ATPase domain and is a key chaperone for thick filament assembly and maintaining its stability. UNC-45 interacts with HSP90 to participate in myosin folding and early myofibril assembly. Lysine methyltransferase Smyd1 acts on HSP90/UNC-45/myosin complex to maintain sarcomere stability. Abnormal protein misfolding caused by stressors is accompanied by increased calpain activity, resulting in excessive protein hydrolysates. In hypertrophic cardiomyopathy, ubiquitin-proteasome system and autophagy-lysosomal system activities are down-regulated. These factors ultimately lead to the toxic level of protein aggregation, which may lead to sarcomere structural disorder and mechanical dysfunction common in failed myocardium.