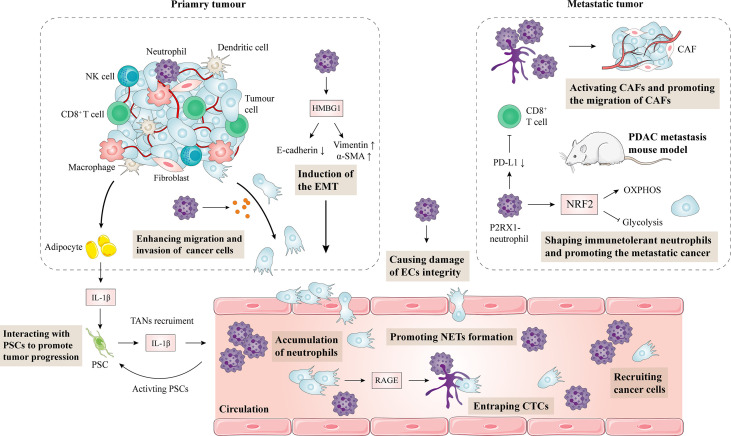
Figure 2.
Impact of neutrophils on the progression and metastasis of pancreatic cancer. Obesity-induced inflammation and TAN infiltration activate PSCs, leading to connective tissue proliferation in the TME and promoting tumor growth. Conversely, PSCs can also recruit TANs. Neutrophils produce HMGB1 in pancreatic cancer, which induces the epithelial-mesenchymal transformation of pancreatic cancer. Moreover, neutrophils can also secrete elastase to degrade E-cadherin on pancreatic cancer cells, resulting in the enhanced migration and invasion of pancreatic cancer cells. In a mouse metastatic tumor model, NRF2 activity in P2RX1 negative neutrophils is elevated, leading to metabolic reprogramming during polarization. As a result, CD8+ T cells are inhibited, and tumor immune escape is mediated. NETs are upregulated in pancreatic cancer through a RAGE dependent and autophagy mediated pathway. NETs enhance the migration of hepatic stellate cells, activate cancer-associated fibroblasts, and promote hepatic metastasis of pancreatic cancer. Neutrophils are also involved in pancreatic cancer vascular endothelial cell integrity damage and promote metastasis of pancreatic cancer cells. CAF, cancer-associated fibroblasts; EMT, epithelial-mesenchymal transformation; PDAC, pancreatic ductal adenocarcinoma; PD-L1, programmed cell death-ligand 1; PSC, pancreatic stellate cell; IL, interleukin; EC, endothelial cell; NET, neutrophil extracellular trap.