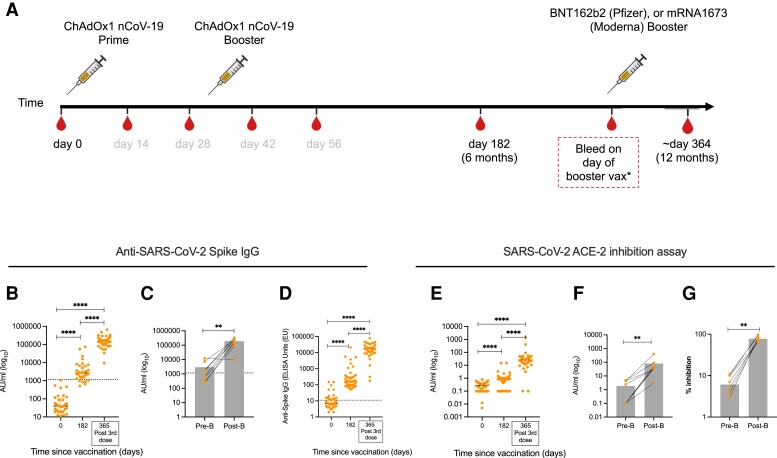
Figure 1.
Anti-SARS-CoV-2 antibody responses are boosted following third dose of COVID-19 vaccines in PWH. A, Vaccination schedule for all participants showing timepoints where samples were used for this study in black. PWH received either BNT162b2, mRNA1273, or ChAdOx1 nCoV-19 vaccines. The third dose was given as close to 1 y after the first vaccine dose as possible. The “Day 365” visit sample was the “post-third dose” sample”. B, Anti-SARS-CoV-2 spike IgG antibody titers before priming vaccine dose at day 0 and post-prime doses at day 182 and 365 (after third dose). C, Anti-SARS-CoV-2 spike IgG antibody titers in HIV positive participants with pre- and post-third dose timepoints. D, In-house ELISA showing anti-spike IgG responses at baseline, day 182 and after third dose (E) ACE-2 inhibition assay on day 0, 182, and after third dose in all participants. F and G, ACE-2 inhibition assay in participants with pre- and post-third dose timepoints expressed as F, AU/mL or G, % inhibition. Comparison of 2 timepoints within the same group was done by Wilcoxon matched pair sign ranked test. Where indicated * = P <.05, ** = P <.01, *** = P < .001 and **** = <.000. “Pre-B” and “Post-B” refer to pre-third dose and post-third dose. Dotted lines indicate cutoff points determined for each SARS-CoV-2 spike antigen based on pre-pandemic sera + 3 × SD. N = 27–33 for HIV positive volunteers in MSD assay. Error bars represent median and interquartile range. Abbreviations: COVID-19, coronavirus disease 2019; ELISA, enzyme-linked immunosorbent assay; HIV, human immunodeficiency virus; IgG, immunoglobulin G; MSD, MesoScale Discovery; PWH, people with HIV; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.