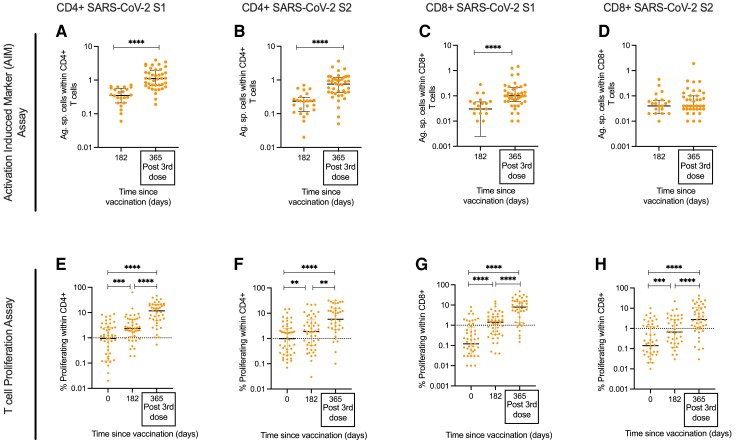
Figure 2.
T-cell response to SARS-CoV-2 following third dose of COVID-19 vaccines in PWH. T-cell responses measured by AIM assay showing magnitude of CD4+ T-cell responses to (A) SARS-CoV-2 S1 and (B) SARS-CoV-2 S2 and magnitude of CD8+ T-cell responses to (C) SARS-CoV-2 S1 and (D) SARS-CoV-2 S2 on days 182 and after third dose (D365). Proliferative T-cell responses assessing kinetics of the T-cell response longitudinally for CD4+ T cells to (E) SARS-CoV-2 S1 and (F) SARS-CoV-2 S2 and CD8+ T cells to (G) SARS-CoV-2 S1 and (H) SARS-CoV-2 S2. Statistical test in (A–D) was done by Mann-Whitney t test. Statistical test in (E–H) was done by Wilcoxon matched pair sign ranked test. Where indicated * = P <.05, ** = P <.01, *** = P < .001 and **** = P <.000. Dotted lines indicate cutoff points determined based on DMSO controls + 3 × SD. n = 24–40 for AIM assay and 41–52 for proliferation assay. Error bars represent median and interquartile range. Abbreviations: AIM, activation induced marker; DMSO, dimethyl sulfoxide; PWH, people with HIV; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation.