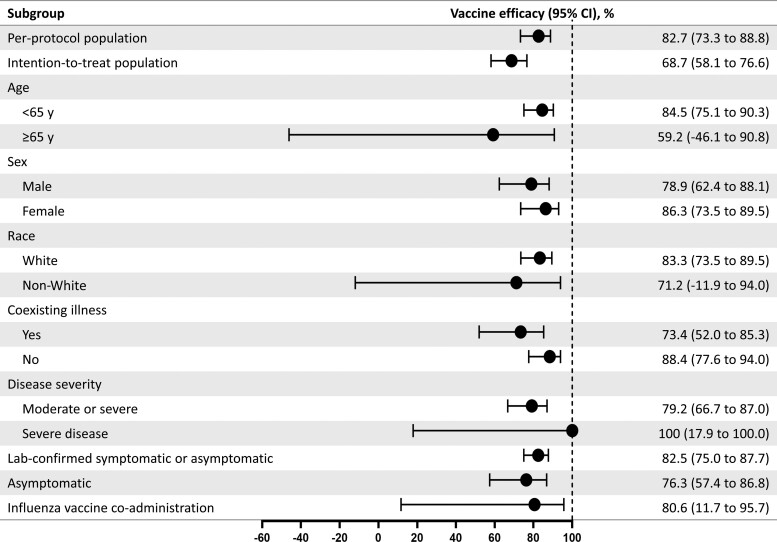
Figure 3.
Vaccine efficacy of NVX-CoV2373 in specific subgroups. The efficacy of NVX-CoV2373 in preventing coronavirus disease 2019 (COVID-19) in various subgroups within the per-protocol population. Vaccine efficacy was defined as 1 minus the relative risk (NVX-CoV2373 vs placebo), and 95% confidence intervals were derived using Poisson regression with robust error variance (except where noted when the Clopper–Pearson exact binomial method was used). Vaccine efficacy for the intention-to-treat population was assessed after dose 1. Data in non-White populations consisted of minority and multiple races, which were pooled to ensure that the subpopulations would be large enough for meaningful analyses. Comorbidity assessment is based on the Centers for Disease Control and Prevention definition of those at increased risk for COVID-19. The laboratory-confirmed symptomatic or asymptomatic and asymptomatic end points are defined in the text. Influenza vaccine coadministration was assessed as part of a predefined influenza vaccine coadministration substudy. Abbreviation: CI, confidence interval.