Abstract
Seizure Related 6 Homolog Like 2 (SEZ6L2) protein has been shown to have implications in neuronal and especially motor function development. In oncology, overexpression of SEZ6L2 serves as a negative prognostic marker in several tumor entities. Recently, few cases of anti-SEZ6L2 antibody mediated cerebellar syndromes were reported. In this article, we present a case of a 70-year-old woman with subacute onset of gait disturbance, dysarthria and limb ataxia. Serum anti-SEZ6L2 antibodies were markedly increased, and further diagnostic workup revealed left sided breast cancer. Neurological symptoms and SEZ6L2 titer significantly improved after curative tumor therapy. This is a very rare and educationally important report of anti-SEZ6L2 autoimmune cerebellar syndrome with a paraneoplastic etiology. Additionally, we performed a review of the current literature for SEZ6L2, focusing on comparing the published cases on autoimmune cerebellar syndrome.
Background
In recent years, several antibodies linked to specific clinical neurological syndromes have been identified [1], indicating the need of a thorough testing for these antibodies in cases with unexplained neurological deficits. This is especially of relevance as these antibody-mediated diseases are treatable. In well-known entities such as anti-NMDAR-encephalitis, the underlying mechanisms are well understood and treatment responses are often favorable [2, 3]. For many other antibody-mediated autoimmune disorders, knowledge regarding origin and treatment options is still lacking.
The Seizure Related 6 (SEZ6) protein family came into the focus of epilepsy research in the 1990s [4]. In the brain, the cell surface protein Seizure Related 6 Homolog Like 2 (SEZ6L2) is a part of the α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, and is highly expressed in the cerebellar cortex, contributing to neuronal growth [5, 6]. Furthermore, in its function as receptor of cathepsin D, SEZ6L2 is a mediator of motor function development [7]. In autism spectrum disorders, mutations in the SEZ6L2 gene were identified [8]. Further research also linked the gene to other psychiatric diseases [9].
Apart from neurosciences, SEZ6L2-expression has also been linked to different types of cancer [10, 11], is associated with poor outcome [12, 13], and therefore can serve as a biomarker [14] and possible therapeutic target [15].
Anti-SEZ6L2 autoimmune cerebellar syndrome was first reported in 2014 [16]. Only a few case reports are available with variable treatment responses. Here, we present the first paraneoplastic case of anti-SEZ6L2 autoimmune cerebellar syndrome caused by breast cancer.
Case report
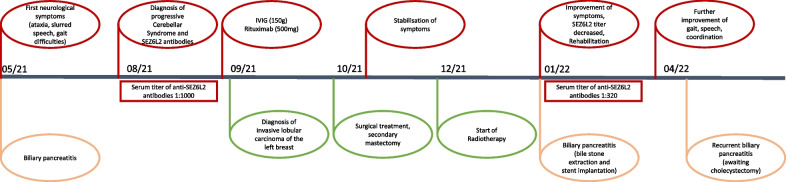
A 70-year-old female presented with slurred speech, ataxia and abnormal gait resulting in multiple falls. Symptoms were progressive and first presented three months prior to admission (Fig. 1). Around that time, the patient suffered from a biliary pancreatitis. Her medical history also included hypertonia treated with beta-blockers and a recently diagnosed depression treated with a selective serotonin reuptake inhibitor (SSRI). There was no family history of neurological disorders.
Fig. 1.
Course of the disease in chronological order
On examination, we found saccadic eye movements and impaired vestibulo-ocular reflex suppression, scanning speech and dysarthria, right sided limb hemiataxia, increased reflexes in the left arm and right leg with ankle clonus and positive Babinski sign. Gait was profoundly impaired by ataxia.
Blood analysis did not indicate a metabolic cause of the cerebellar syndrome. Cerebrospinal fluid (CSF) analysis resulted in normal cell count and protein levels. Oligoclonal bands (OCB) were positive. Phospho-tau was slightly increased to 71 pg/ml (normal range < 61). All other parameters including cytology were normal. However, auto-antibody panel analysis for autoimmune encephalitis/cerebellitis was positive for anti-SEZ6L2 antibodies with a titer of 1:1000 in the serum (reference range: < 1:10). Anti-SEZ6L2 antibodies were detected by immunohistochemistry on cerebellar tissue and were confirmed by specifically transfected human embryonic kidney (HEK) cells. CSF was not tested for anti-SEZ6L2 antibodies due to lack of material.
Brain MRI showed atrophy of the vermis and cerebellar hemispheres (Fig. 2). Spinal MRI revealed no pathologies of the spinal cord.
Fig. 2.
MR image of the patient shows atrophy of cerebellar hemispheres (arrows) in Axial T2-FLAIR images a and coronal T1-weighted images b and vermis atrophy (arrowhead) in sagittal T2-weighted images (c)
The patient was started on a five-day course of intravenous immunoglobulins (IVIG, total dose 150 g) followed by a single cycle of rituximab (500 mg).
Whole body 18F FDG-PET-CT suspected breast cancer. Further work up revealed an invasive lobular carcinoma of the left breast (Fig. 1). The patient was referred to gynecology. Immunohistochemistry revealed estrogene receptor positivity (100%), progesterone receptor positivity (15%), and HER2-neu negativity. After mastectomy including sentinel lymph node excision, adjuvant radiotherapy was performed, followed by aromatase-inhibitor therapy.
At this stage of her treatment, we followed up on the patient. She reported stabilization of speech and gait without further deterioration, this was congruent with the neurological examination. We decided against further cycles of rituximab due to improvement of symptoms.
Rehabilitation was delayed because the patient suffered from another biliary pancreatitis (Fig. 1), treated by bile duct stone extraction and stent implantation. At this point, neurological examination revealed a less severe gait and only slight limb ataxia and the patient reported profound stabilization of gait using a walker as well as improvement of speech fluency and articulation (Fig. 1). Anti-SEZ6L2 antibody titer in the serum decreased to 1:320.
Three month later, after rehabilitation, the patient presented to our outpatient clinic, reporting profound improvement regarding walking distance, speed and coordination and nearly normalization of speech. Neurological examination still showed saccadic eye movements and a left sided ataxia in the arm and leg. Gait was more fluent and secure, although still clearly impaired (Fig. 1). Cholecystectomy was planned due to recurrent pancreatitis.
Published cases of SEZ6L2 associated cerebellar syndrome
Up to now, eight cases of SEZ6L2-associated cerebellar syndrome have been reported in the literature (Fig. 3, Table 1). It was first described 2014 in a 60-year-old female patient who presented with additional retinopathy [16]. Cerebellar syndrome was associated with parkinsonism in five of the eight patients [17]. Cognitive dysfunction was reported in two cases [18, 19]. All but two reported cases had normal standard CSF parameters. In one case, a pleocytosis was found [18], in another case high protein levels [19]. In two cases OCB were examined, with a negative result [17, 18]. Positive OCB, as observed in our patient, were not reported. Abnormalities of neurodegenerative markers were reported in two cases [17, 19]. Brain MRI of a few of the cases showed cerebellar atrophy. One case with predominantly cognitive function abnormalities presented with hippocampal atrophy [19]. An underlying malignancy proposing a paraneoplastic mechanism was found in a patient years after onset of cerebellar symptoms [18]. In another report, small cell lung cancer (SCLC) was diagnosed after onset of neurological symptoms [19]. Immunosuppressive therapy was applied in all patients. The outcome was mostly unfavorable. A positive outcome was only reported in two of the eight cases, one after receiving cyclophosphamide [20], the other after receiving an immunotherapy not further specified in the paper [16]. The patient with SCLC received immunosuppressive therapy as well as cancer treatment and died after five months. Our patient improved after breast cancer treatment, preceded by IVIG treatment and a single dose of Rituximab.
Fig. 3.
Review of published literature on SEZ6L2
Table 1.
Review of published cases of SEZ6L2 associated cerebellar syndrome
| References | Cases (comorbidities if specified) | Symptoms | Diagnostic findings | Treatment | Outcome |
|---|---|---|---|---|---|
| Yaguchi et al. [16] | 60yo F | Severe ataxia, retinopathy |
CSF: normal MRI brain: normal (cerebellar atrophy after 2 y) No malignancy found |
Immunotherapy (no further specification given) | Mild improvement (24 months follow up) |
| Borsche et al. [17] | 55yo F with Crohn’s disease |
Square wave jerks Limb Ataxia Impaired gait Postural instability |
CSF: normal cell count and protein levels, OCB negative, beta-amyloid levels decreased, normal tau levels MRI brain: cerebellar atrophy PET-CT: normal |
IVIG Rituximab |
Deterioration in spite of IVIG, improvement after Rituximab (12 month follow up) |
| Landa et al. [18] | 69yo M | Dysarthria, gait ataxia, headache, postural instability, apraxia, echolalia, axial rigidity, hypomimia, bradykinesia, hypophonia, diplopia, saccadic eye movements |
CSF: Pleocytosis (90/µl), normal glucose and protein levels, OCB negative MRI brain: normal PET-CT: normal |
IVMP IVIG Rituximab Cyclophosphamide |
Further deterioration (10 months follow up) |
| 55yo F | Dysarthria, gait ataxia, limb ataxia, cognitive impairment, unilateral parkinsonism, downbeating and torsional nystagmus |
CSF: normal MRI brain: normal Metastatic ovarian cancer (4 years after onset auf cerebellar syndrome) |
IVMP IVIG |
Further deterioration (death after 52 months from ovarian cancer) | |
| 54yo M | Dysarthria, gait ataxia, limb ataxia, mild cognitive impairment, bradykinesia, saccadic eye movements, end-gaze nystagmus |
CSF: normal MRI brain: normal No malignancy found |
IVMP Plasmapheresis |
Further deterioration (36 months follow up) | |
| 69yo F | Dysarthria, gait ataxia, limb ataxia, downbeat nystagmus |
CSF: normal MRI brain: normal No malignancy found |
Prednisone Cyclophosphamide |
Further deterioration (72 months follow up) | |
| Mehdiyeva et al. [20] | 73yo F with depression |
Nausea Bilateral gaze-evoked nystagmus, dysarthria, truncal ataxia, Postural instability, hypophonia, bradykinesia |
MRI brain: cerebellar atrophy PET-CT: normal |
IVMP IVIG Rituximab Cyclophosphamide |
Marked improvement with cyclophosphamide (15 months follow up) |
| Carneiro et al. [19] | 62yo F with mild hyponatremia, hypothyroidism |
Gait ataxia, limb ataxia Disorientation, anterograde memory loss |
CSF: high protein (87 mg/dL), high tau (2130 pg/mL, reference < 335 pg/mL) MRI: hippocampal atrophy, T2-hyperintensity of right hippocampus PET-CT: lung carcinoma suspected, confirmed by further evaluation |
IVMP IVIG Cancer treatment |
Death after 5 months |
| Our case 2022 | 70yo F with recurrent biliary pancreatitis, depression | saccadic eye movements, disrupted vestibulo-ocular reflex, scanning speech and dysarthria, right sided limb hemiataxia, increased reflexes in the left arm and right leg with ankle clonus and pyramidal signs, profound gait ataxia |
CSF: normal cell count and protein levels, OCB positive, phospho-tau increased (71 pg/ml, normal range < 61) MRI brain: cerebellar atrophy PET-CT: Mamma Ca suspected, confirmed by further evaluation |
IVIG Rituximab (once) Cancer treatment |
Improvement after mastectomy (8 months follow up) |
yo years old, F female, M male, CSF cerebrospinal fluid, MRI magnet resonance imaging, OCB oligoclonal bands, PET-CT positron emission tomography computer tomography, IVIG intravenous immunoglobulins, IVMP intravenous methylprednisolone
SEZ6L2 in other clinical contexts
Apart from autoimmune cerebellar syndrome, SEZ6L2 also plays a role in other clinical contexts in neurology and psychiatry (Fig. 3).
In an animal study using knock-out mice, SEZ6L2 was found to have an influence on motor skill and coordination development [21]. SEZ6L2 has been proposed as a CSF biomarker differentiating idiopathic normal pressure hydrocephalus from Alzheimer’s disease [22]. High gene expression levels of SEZ6L2 in patients with glioblastoma were found to be a negative prognostic factor [12]. In degenerative disc disease, high gene expression of SEZ6L2 has been associated with an inflammatory etiology [23]. Mutation in the SEZ6L2 gene and the broader family of SEZ6 proteins are also in the focus of research on autism [24], febrile seizures in children [25, 26], bipolar disorder [9] and schizophrenia [27].
Outside the neurological and psychiatric field, high SEZ6L2 gene expression in tumor tissue is a negative prognostic factor in various tumor entities. Specifically, overexpression of SEZ6L2 has been linked to an unfavorable outcome in glioblastoma [12], colorectal cancer [28, 29], cholangiocarcinoma [13], lung adenocarcinoma [15], non-small cell lung cancer [10], thyroid cancer [30], hepatocellular carcinoma [31], and breast cancer [11]. In ovarian cancer, SEZ6L2 was shown to be a serological biomarker [14]. For osteosarcoma, an upregulation of SEZ6L2 was associated with methylation [32].
Interestingly, in a mouse model of lung adenocarcinoma, anti-SEZ6L2 antibodies had a positive effect on drug resistance and metastasis [15].
Apart from being upregulated in malignancies, SEZ6 proteins play a role in complement regulation [33] and in the pancreas, SEZ6L2 is specific to islet cells [34].
Discussion
With our case report, we add knowledge to the newly discovered entity of anti-SEZ6L2 mediated autoimmune cerebellar syndrome. Uniquely, we detected a paraneoplastic origin of this entity due to breast cancer. Symptoms markedly improved after curative cancer therapy. Interestingly, our patient suffered from biliary pancreatitis recurrently during the cerebellar syndrome. As SEZ6L2 is a marker of pancreatic islet cells [34], a link to a predisposition to pancreatitis with increased anti-SEZ6L2 antibodies in the body could be speculated.
There is evidence for direct pathogenicity of the anti-SEZ6L2 antibodies in the development of cerebellar syndrome [5]. Nonetheless, considering the various roles SEZ6L2 plays as part of the AMPA receptor [6] and in cathepsin D transport [7], a degenerative mechanism could also be involved. Further research is needed to address this question.
The paraneoplastic origin of the cerebellar syndrome observed in our patient is especially interesting in the light of the prognostic value of SEZ6L2 expression in various types of cancer [10–15, 28–31]. In the future, anti-SEZ6L2 treatment might be possible to positively influence the course of malignancies [15]. As neurologists experienced with side effects of checkpoint inhibitors [35], this approach could also trigger autoimmune side effects resulting in encephalitis or cerebellitis. Therefore, SEZ6L2 antibody mediated autoimmune cerebellar syndrome should be on the list of differential diagnosis for subacute ataxia now and in the future.
Acknowledgements
Not applicable.
Author contributions
AK, FH, RB, SCT and JBS treated the patient during her inpatients and outpatients visits. AK analysed and interpreted the clinical data and performed the review of the literature with close supervision by SCT. DH and MW performed MRI of the brain and designed Fig. 2. KPW and RM performed laboratory analysis for the antibody. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Given by the patient.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Uy CE, Binks S, Irani SR. Autoimmune encephalitis: Clinical spectrum and management. Practical Neurology. 2021;21:412–423. doi: 10.1136/practneurol-2020-002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaler FS, et al. Rituximab treatment and long-term outcome of patients with autoimmune encephalitis: Real-world evidence from the GENERATE Registry. Neurology Neuroimmunology Neuroinflammation. 2021;8:e1088. doi: 10.1212/NXI.0000000000001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nosadini M, et al. Use and safety of immunotherapeutic management of N-methyl-d-aspartate receptor antibody encephalitis: A meta-analysis. JAMA Neurology. 2021;78:1333–1344. doi: 10.1001/jamaneurol.2021.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimizu-Nishikawa K, Kajiwara K, Kimura M, Katsuki M, Sugaya E. Cloning and expression of SEZ-6, a brain-specific and seizure-related cDNA. Molecular Brain Research. 1995;28:201–210. doi: 10.1016/0169-328X(94)00203-Q. [DOI] [PubMed] [Google Scholar]
- 5.Yaguchi H, et al. Anti-Sez6l2 antibody detected in a patient with immune-mediated cerebellar ataxia inhibits complex formation of GluR1 and Sez6l2. Journal of Neurology. 2018;265:962–965. doi: 10.1007/s00415-018-8785-z. [DOI] [PubMed] [Google Scholar]
- 6.Yaguchi H, et al. Sez6l2 regulates phosphorylation of ADD and neuritogenesis. Biochemical and Biophysical Research Communications. 2017;494:234–241. doi: 10.1016/j.bbrc.2017.10.047. [DOI] [PubMed] [Google Scholar]
- 7.Boonen M, et al. Cathepsin D and its newly identified transport receptor SEZ6L2 can modulate neurite outgrowth. Journal of Cell Science. 2016;129:557–568. doi: 10.1242/jcs.179374. [DOI] [PubMed] [Google Scholar]
- 8.Konyukh M, et al. Variations of the candidate SEZ6L2 gene on Chromosome 16p11.2 in patients with autism spectrum disorders and in human populations. PLoS ONE. 2011;6:e17289. doi: 10.1371/journal.pone.0017289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maccarrone G, et al. Psychiatric patient stratification using biosignatures based on cerebrospinal fluid protein expression clusters. Journal of Psychiatric Research. 2013;47:1572–1580. doi: 10.1016/j.jpsychires.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa N, et al. Characterization of SEZ6L2 cell-surface protein as a novel prognostic marker for lung cancer. Cancer Science. 2006;97:737–745. doi: 10.1111/j.1349-7006.2006.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Han S, Li Y, Zheng Y, Zhang Q. SEZ6L2, regulated by USF1, accelerates the growth and metastasis of breast cancer. Experimental Cell Research. 2022;417:113194. doi: 10.1016/j.yexcr.2022.113194. [DOI] [PubMed] [Google Scholar]
- 12.Yu Z, Du M, Lu L. A novel 16-genes signature scoring system as prognostic model to evaluate survival risk in patients with glioblastoma. Biomedicines. 2022;10:317. doi: 10.3390/biomedicines10020317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, et al. Overexpression of SEZ6L2 predicts poor prognosis in patients with cholangiocarcinoma. Translational Cancer Research. 2020;9:6768–6779. doi: 10.21037/tcr-20-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dufresne J, et al. The plasma peptides of ovarian cancer. Clinical Proteomics. 2018;15:41. doi: 10.1186/s12014-018-9215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J-S, et al. SEZ6L2 is an important regulator of drug-resistant cells and tumor spheroid cells in lung adenocarcinoma. Biomedicines. 2020;8:500. doi: 10.3390/biomedicines8110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaguchi H, et al. Identification of anti-Sez6l2 antibody in a patient with cerebellar ataxia and retinopathy. Journal of Neurology. 2014;261:224–226. doi: 10.1007/s00415-013-7134-5. [DOI] [PubMed] [Google Scholar]
- 17.Borsche M, et al. Sez6l2-antibody-associated progressive cerebellar ataxia: A differential diagnosis of atypical parkinsonism. Journal of Neurology. 2019;266:522–524. doi: 10.1007/s00415-018-9115-1. [DOI] [PubMed] [Google Scholar]
- 18.Landa J, et al. Seizure-related 6 homolog like 2 autoimmunity: Neurologic syndrome and antibody effects. Neurology-Neuroimmunology Neuroinflammation. 2021;8:e916. doi: 10.1212/NXI.0000000000000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis Carneiro D, Maresch Â, Cunha R, Morgadinho A. Sez6l2-associated encephalitis in a patient with small-cell lung cancer. Neurological sciences: Official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2022 doi: 10.1007/s10072-022-06244-z. [DOI] [PubMed] [Google Scholar]
- 20.Mehdiyeva A, Hietaharju A, Sipilä J. SEZ6L2 antibody-associated cerebellar ataxia responsive to sequential immunotherapy. Neurology-Neuroimmunology Neuroinflammation. 2022;9:e1131. doi: 10.1212/NXI.0000000000001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash A, et al. Lack of Sez6 family proteins impairs motor functions, short-term memory, and cognitive flexibility and alters dendritic spine properties. Cerebral Cortex. 2020;1991(30):2167–2184. doi: 10.1093/cercor/bhz230. [DOI] [PubMed] [Google Scholar]
- 22.Torretta E, et al. Novel insight in idiopathic normal pressure hydrocephalus (iNPH) biomarker discovery in CSF. International Journal of Molecular Sciences. 2021;22:8034. doi: 10.3390/ijms22158034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang N, et al. Identification of SMIM1 and SEZ6L2 as potential biomarkers for genes associated with intervertebral disc degeneration in pyroptosis. Disease Markers. 2022;2022:9515571. doi: 10.1155/2022/9515571. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Kumar RA, et al. Association and mutation analyses of 16p11.2 autism candidate genes. PLoS ONE. 2009;4:e4582. doi: 10.1371/journal.pone.0004582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Z-L, et al. Febrile seizures are associated with mutation of seizure-related (SEZ) 6, a brain-specific gene. Journal of Neuroscience Research. 2007;85:166–172. doi: 10.1002/jnr.21103. [DOI] [PubMed] [Google Scholar]
- 26.Mulley JC, et al. The role of seizure-related SEZ6 as a susceptibility gene in febrile seizures. Neurology Research International. 2011;2011:917565. doi: 10.1155/2011/917565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambalavanan A, et al. De novo variants in sporadic cases of childhood onset schizophrenia. European Journal of Human Genetics EJHG. 2016;24:944–948. doi: 10.1038/ejhg.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An N, et al. SEZ6L2 knockdown impairs tumour growth by promoting caspase-dependent apoptosis in colorectal cancer. Journal of Cellular and Molecular Medicine. 2020;24:4223–4232. doi: 10.1111/jcmm.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu J, Chen Q. Transcriptome-based identification of molecular markers related to the development and prognosis of Colon cancer. Nucleosides, Nucleotides & Nucleic Acids. 2021;40:1114–1124. doi: 10.1080/15257770.2021.1975298. [DOI] [PubMed] [Google Scholar]
- 30.Luo X, et al. High expression of SEZ6L2 as an independent prognostic Indicator in thyroid carcinoma. Gland Surgery. 2022;11:412–425. doi: 10.21037/gs-22-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, et al. Upregulated seizure-related 6 homolog-like 2 is a prognostic predictor of hepatocellular carcinoma. Disease Markers. 2020;2020:7318703. doi: 10.1155/2020/7318703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q. CpG methylation patterns are associated with gene expression variation in osteosarcoma. Molecular Medicine Reports. 2017;16:901–907. doi: 10.3892/mmr.2017.6635. [DOI] [PubMed] [Google Scholar]
- 33.Qiu WQ, et al. The Sez6 family inhibits complement by facilitating factor I cleavage of C3b and accelerating the decay of C3 convertases. Frontiers in Immunology. 2021;12:607641. doi: 10.3389/fimmu.2021.607641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hald J, et al. Pancreatic islet and progenitor cell surface markers with cell sorting potential. Diabetologia. 2012;55:154–165. doi: 10.1007/s00125-011-2295-1. [DOI] [PubMed] [Google Scholar]
- 35.Velasco R, et al. Encephalitis induced by immune checkpoint inhibitors: A systematic review. JAMA Neurology. 2021;78:864–873. doi: 10.1001/jamaneurol.2021.0249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.