Abstract
Background
Schistosomiasis is known to affect the cognitive functions of children, however, but there is paucity of information on its impact on early childhood development in developing countries where the disease is endemic. This study aimed at determining the effects of schistosomiasis due to Schistosoma haematobium on early childhood development in children below 5 years old from Murewa District, Zimbabwe, including the benefits of treatment.
Methods
Preschool age children (PSAC) under the age of 5 years were screened at baseline and at 6 months post-treatment for S. haematobium infections diagnosed using the urine filtration method. Cognitive domains were assessed using the Griffith Mental Developmental Scales III on 136 PSAC. Multivariate logistic regression was used to determine the level of association between S. haematobium infection and performance in the cognitive domains adjusting for confounding factors (i.e. nutrition, hemoglobin levels, gender and age). Median Development Quotient scores of each cognitive domain at baseline and at 6 months post-treatment were compared and quantified.
Results
After adjusting for confounding factors, PSAC infected with S. haematobium had greater odds of having lower scores in the Foundation of Learning Domain (OR = 3.9, p = 0.008), Language and Communication Domain (OR = 3.2, p = 0.017), Eye-Hand Coordination Domains (OR = 10.7, p = 0.001), Personal-Social-Emotional Domain (19.3, p = 0.001) and in the Overall General Development Domain (7.2, p = 0.011). Improvement of cognitive performance was observed at 6 months post treatment in the following Domains; Language and Communication Domain (p = 0.003), Eye-Hand Coordination Domain (p = 0.02) and General Development Domain (p = 0.006).
Conclusion
The study showed that S. haematobium infection in PSAC is associated with lower cognitive scores in the Foundation of Learning, Language and Communication, Eye-Hand Coordination, Personal-Social-Emotional and in the Overall General Development domains. Our results strengthen the call for inclusion of PSAC in routine deworming programs for the control of urinary schistosomiasis and the need to develop locally validated tools to monitor early child development in endemic areas where resources are limited.
Keywords: Cognitive functions, Early child development, Pre-school aged children, Schistosomiasis
Introduction
Children growing up in schistosomiasis endemic areas have been disproportionately affected by the disease for decades, as they account for 123 million of the total global burden of over 250 million people infected [1]. Schistosomiasis affects multiple body systems such as the urogenital, gastrointestinal, respiratory and nervous system [2–5]. The impact of infection within the nervous system has been linked to learning difficulties, poor school performance, growth retardation and cognitive deficits [6–8]. The majority of the infected children reside in low-income countries particularly in regions that have poor water supply and sanitation facilities [9–11].
In an effort to reduce schistosomiasis morbidity, national deworming programs have targeted school aged children above the age of five years who are at risk of infection (12). However, to date pre-school age children (PSAC) under the age of five years who are also exposed to infection are excluded from the routine deworming programs and are only treated after confirmed diagnosis [12, 13]. Infection control is hindered in this age group due to existing gaps in research on exposure patterns, unavailability of a paediatric praziquantel formulation [14], impact of infection and the absence of the true global burden of schistosomiasis in the PSAC age group [13]. Inclusion of PSAC as the target population on mass drug administration programs for schistosomiasis treatment can be justified by providing evidence of the negative developmental effects of schistosomiasis infections on cognitive domains [15].
According to the Canadian Council on learning (2010); cognitive development in the first five years of early childhood development is crucial as it has an impact on the future success of the child in school, workplace as an adult, and many other aspects of a healthy fulfilling life. The first five years of childhood is characterized by rapid growth and development of the brain [16]. A meta-analysis conducted in 2018 reported on the negative associations of schistosomiasis and cognitive deficits in school aged going children, particularly in the educational, learning and memory domain [8]. However, to date there is paucity of research that shows the direct and indirect health developmental impact of schistosomiasis on PSAC including the benefits of treatment in this age group. Although the mechanism of how schistosomiasis causes cognitive deficits is unknown, it has been suggested that inflammatory mediators could be the cause of disturbances in the hippocampal neuronal functions and hence affecting working memory consolidation [17–20].
This study thus aimed to bridge the knowledge gap by determining the effect of S. haematobium infections on early childhood development and cognitive domains in order to provide substantial evidence base to promote comprehensive national programs for schistosomiasis control in the PSAC age group.
Methods
Ethical approval
Permission was granted by the Medical Research Council of Zimbabwe (MRCZ/A/2246 and MRCZ/A/2573), Provincial and District Medical Directors and parents of the participating children. The study goals and methodology were explained in the local language (chiShona) to parents and guardians and written parental consent was acquired prior to recruitment of the PSAC. Recruitment of participants was on a voluntary basis and PSAC were allowed to withdraw at any time during the study. Treatment was administered by a clinician and the children were closely monitored for 4 h post treatment.
Study area
The study was conducted in Magaya village (Murewa district) which is located in Mashonaland East province of Zimbabwe (17°38′49″S 31°46′39″E). The population in Murewa district is 199,607 and subsistence farming is the main activity in this area and contact with unsafe water is frequent (assessed by questionnaire) due to inadequate and poor safe water facilities. The area is reported to have high S. haematobium prevalence (> 50%), low S. mansoni and soil transmitted helminths prevalence (< 2%) and low S. haematobium and S. mansoni co-infections have been reported [11]. Exposure of the village population to S. haematobium infections is through washing, gardening, bathing and collection of water from infected snail infested water sources whilst most of the PSAC are exposed to infections passively (mothers and guardians take their PSAC to infected water contact points while doing their water related chores).
Study Design and Population
The case control study was conducted on PSAC who were matched according to gender and age at baseline to investigate the effect of S. haematobium infections on the cognitive functions of PSAC. The study also employed a 6-month post-treatment follow up on the cases to assess the possible cognitive improvement after treatment. A case was defined as a pre-school aged child who had at least one S. haematobium egg in their urine samples following screening with the urine filtration technique and microscopy [21]. Controls were defined as PSAC who did not have S. haematobium or S. mansoni eggs detected in their urine or fecal samples, respectively. A minimum sample size of 133 (27 cases and 106 controls) was calculated to report 80% power and 95% confidence interval assuming 50% exposure in cases and 22% exposure in controls using Odds Ratio of 3.5.
Inclusion criteria
One hundred and thirty-six PSAC who met all of the following seven inclusion criteria were enrolled in the study from primary health centers in Magaya, Murewa district; (i) were lifelong residents of Magaya, Murewa district (ii) have never been given anthelminthic treatment for schistosomiasis (iii) were less than 5 years at baseline recruitment (iv) did not have mental and/or physical disabilities (v) did not have S. mansoni and/or soil transmitted helminths infection after screening with the Kato-Katz method (vii) written consent obtained from parent to participate in the study. Eleven PSAC (8%) who had developmental challenges (DQ scores below 50) were excluded from the study and referred to a clinical psychologist for further examination and therapy.
Collection of blood and determination of hemoglobin levels
A clinician collected approximately 5 ml of blood from each PSAC into vacutainer tubes that contained EDTA anticoagulant (BD vacutainer, Fisher Scientific). Blood samples were placed in insulated ice cooler boxes and processed in the laboratory within 4 h. A hematology analyzer (MaxM; Coulter, Fullerton, CA) was used to measure the hemoglobin concentration levels in the blood.
Anthropometry
The height for age index and weight for age index was used to classify the nutritional status of the PSAC using the WHO child growth standards. Height measurements rounded off to the nearest centimetre (cm) were taken using a stadiometer (Gima®). Each PSAC was weighed in light clothes using an electronic scale (Gima®). Measurement of the mid-upper arm circumference (MAUC) for the diagnosis of malnutrition was done using a MAUC tape (AnthroFlex®). The anthropometric measurements were used to generate Z-scores for the Height-for-Age and Weight-for-Age index using the WHO Anthro software, version 3.0.1(http://www.who.int/childgrowth/en/). PSAC with weight Z-scores < -2 were classified as underweight and if they had Height-for-Age Z-scores < -2 they were classified as stunted.
Parasitology analysis
Urine and stool samples were collected from each participant over three consecutive days and examined microscopically for the presence of S. haematobium eggs using the urine filtration method and the Kato-Katz technique for the detection of S. mansoni and soil transmitted helminth eggs [21, 22]. PSAC were classified as infected if at least one S. haematobium or S. mansoni egg was detected in the urine or stool sample, respectively. PSAC who were found to be infected were treated with praziquantel (PZQ) at a standard dosage of 40 mg/kg body weight by a local clinician.
Psychological examination
The Griffiths Mental Developmental Scales III for children below 72 months (6 years) was used in this study [23, 24]. The scales measured by this tool were as follows; Eye and Hand Coordination, Personal-Social-Emotional, Language and Communication, Foundations of Learning, Gross Motor Function and Overall General Development. The PSAC were assessed by an experienced clinician who had completed an accredited training course on the Griffiths scales. Developmental Quotient (DQ) scores were calculated using the raw data from each scale and further categorized as dichotomous categorical variables for analysis using the linear regression model. Each scale was analyzed separately in which low performance was categorized for DQ scores less than 88.
Statistical analyses
Stata Version 16.0 (StataCorp LLC, Texas, USA) was used to analyze the data which was first checked and adjusted for normality. T-test and the Mann Whitney test were used for the analysis of the parametric and non-parametric continuous DQ scores respectively. Multivariate Logistic regression analysis was done to determine the association between S. haematobium infection and performance on the cognitive domains. The categorized DQ scores for each cognitive domain were used as dependent variables while infection status was used as the independent variable in the logistic regression model. Each confounding factor (nutritional status, age, gender and hemoglobin levels) were analyzed separately as independent variables in the logistic regression model to determine their independent associations with each cognitive domain. The Wilcoxon matched-pairs signed rank test was used to compare the continuous variable DQ scores of infected children at baseline and at 6 months post treatment timeline. Results with p-values < 0.05 were considered to be statistically significant.
Results
Characteristics of the study population at baseline before schistosomiasis treatment is shown in Table 1. One hundred and thirty-six PSAC were screened for S. haematobium infections and 30 (22%) PSAC were diagnosed with S. haematobium infections and none with S. mansoni and soil transmitted helminths at baseline. Most of the PSAC (86.7%) had moderate infection intensity with 13.5 mean egg count/10 ml urine of urine. The study had more infected female PSAC (22%) than male PSAC (8%). At 6 months post treatment, only five PSAC had S. haematobium re-infections.
Table 1.
Demographic characteristics, infection status with Schistosoma haematobium and anthropometry of pre-school aged children from Murewa District, Zimbabwe, who participated in a six-month follow-up study
| Variable | Whole cohort (n = 136) |
Uninfected (n = 106) |
Infected (n = 30) |
P- value |
|---|---|---|---|---|
|
Foundations of Learning DQ Mean (SD) |
95.6 (18.9) | 97.6 (19.1) | 88.4 (16.8) | 0.0176 |
|
Language and Communication DQ Median (IQR) |
96.3 (20) | 98.9 (19.7) | 94.2 (21.1) | 0.5268 |
|
Eye-Hand `Coordination DQ Mean (SD) |
101.7 (15.1) | 102.7 (14.7) | 98.2 (16.1) | 0.1470 |
|
Personal-Social-Emotional DQ Median (IQR) |
107.7 (19) | 109.1 (19) | 105.5 (23) | 0.2324 |
|
Gross Motor Function DQ Mean (SD) |
107.7 (18.7) | 112 (16) | 109.5 (25) | 0.2667 |
|
Overall General Development DQ Mean (SD) |
102.2 (12.5) | 103.7 (11.9) | 97 (13.3) | 0.0085 |
|
Age (months) Median (IQR) |
51 (23) | 50 (25) | 52 (20) | 0.0803 |
|
BMI Z-score Median (IQR) |
-0.63 (1.26) | -0.66 (-1.2) | -0.32 (-1.00) | 0.3808 |
|
Hemoglobin Median (IQR) |
12 (1.1) | 12.1 (1.1) | 12 (1) | 0.0270 |
|
S. haematobium infection mean eggs/10ml urine (SD) |
0 | 13.5(28.5) | ||
| % | % | % | P value | |
| Sex: | ||||
| Male | 73 | 61.32 | 26.67 | 0.001 |
| Female | 63 | 38.68 | 73.3 |
SD = standard deviation, IQR = interquartile range, g/dl = grams per deciliter, P-value in bold signifies significance at P < 0.05
Foundations of Learning Domain
PSAC with S. haematobium infection were 3.9 times more likely to have low performance in the Foundations of Learning domain in comparison to uninfected PSAC (p = 0.008) (Table 2). Age was found to be a significant confounder affecting this domain (p < 0.05) indicating that older PSAC were less likely to have lower Foundations of Learning scores than the younger PSAC. The other potential confounders (gender, nutritional status and hemoglobin levels) had no significant associations with PSAC performance in the Foundations of Learning.
Table 2.
Multivariate analysis results of Griffiths Cognitive Domains of gender, hemoglobin and age on pre-school aged children uninfected and infected with Schistosoma haematobium
| Variable | FLDQ | LCDQ | PSEDQ | EHCDQ | GDDQ | |
|---|---|---|---|---|---|---|
| AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | AOR (95% CI) | ||
| Gender |
Male p value |
2.4 (0.6–9.2) 0.207 |
0.6 (0.1–2.4) 0.432 |
2.6 (0.4–17.7) 0.318 |
19.6 (1.1-356.2) 0.044 |
0.5 (0.6–3.6) 0.469 |
| Nutritional status | p-value |
1 (0.5–1.8) 0.976 |
0.6 (0.3–1.2) 0.155 |
1.2 (0.4–3.2) 0.718 |
0.4 (0.2–1.1) 0.071 |
0.9 (0.4-2.0) 0.776 |
| Hemoglobin | p-value |
0.9 (0.4–1.9) 0.753 |
0.3 (0.11–0.8) 0.014 |
2.5 (0.8–7.5) 0.114 |
1.5 (0.4–5.4) 0.500 |
0.3 (0.1–1.1) 0.068 |
| Age | p-value |
0.9 (0.9-1.0) 0.003 |
0.9 (0.9-1.0) 0.023 |
0.9 (0.8-1.0) 0.061 |
0.9 (0.8-1.0) 0.03 |
0.9 (0.9-1.0) 0.996 |
|
S. haematobium Infection status |
Infected p-value |
4.6 (1.2–17.2) 0.023 |
3.6 (1.0-13.1) 0.047 |
5.9 (0.9–41) 0.074 |
28.2 (2.0-414.6) 0,015 |
5.3 (0.7–36) 0.090 |
FLDQ = Foundations of Leaning Development Quotient, LCDQ = Language and Communication Development Quotient, PSEDQ = Personal-Social-Emotional Development Quotient, EHCDQ = Eye and Hand Coordination Development Quotient, GDDQ = General Development Quotient AOR = adjusted odds ratio; CI = confidence interval; All AORs quantify the odds of poor performance on cognitive domains adjusted for the effect of confounders (age, hemoglobin level, and nutritional status). Statistical significance is indicated by bold numbers. Reference categories for the gender category are females and for the S haematobium infection category are uninfected PSAC
Language and Communication Domain
PSAC with S. haematobium infections had 3.2 odds of lower performance in the Language and Communication Domain (p = 0.017) in comparison to uninfected PSAC (Table 2). Age was also found to be significant confounders (p = 0.016) affecting cognitive performance in this domain. Older PSAC were at lesser odds (OR = 0.9) of having lower Language and Communication scores than the younger PSAC. Nutritional status, hemoglobin levels and gender had no significant effects on performance in the Language and Communication Domain.
Eye and Hand Coordination Domain
PSAC infected with S. haematobium had 10.7 times odds of lower performance in the Eye and Hand Domain in comparison to uninfected PSAC (p = 0.001) (Table 2). Age, hemoglobin levels and gender were significant confounders affecting performance in this domain. Older PSAC were at lesser odds (OR = 0.9) of having low scores in the Eye and Hand Coordination Domain. Males PSAC had higher odds (4.9) of having low scores compared to the females. Nutritional status and hemoglobin levels had no significant association with performance in the Eye and Hand Coordination Domain.
Personal-Social-Emotional Domain
PSAC infected with S. haematobium had 19.3 odds of lower performance in comparison to uninfected PSAC (Table 2). Older children had lesser odds (0.9) of performing lower in this domain compared to the younger PSAC. Gender, nutritional status, and hemoglobin levels had no significant associations with performance in this domain.
Gross Motor Function Domain
The nutritional status of PSAC was a confounder affecting performance in this domain (Table 2). PSAC with lower weight for age scores had greater odds of having lower scores in the Gross Motor Function Domain. Infection status, hemoglobin levels, age and gender had no significant associations with the Gross Motor Function Domain.
Overall General Development
PSAC with S. haematobium infections had their Overall General Development affected, as they had 7.2 odds of lower General Development in comparison to the uninfected PSAC. Older PSAC had lesser odds of having low overall General Development compared to the younger PSAC (Table 2).
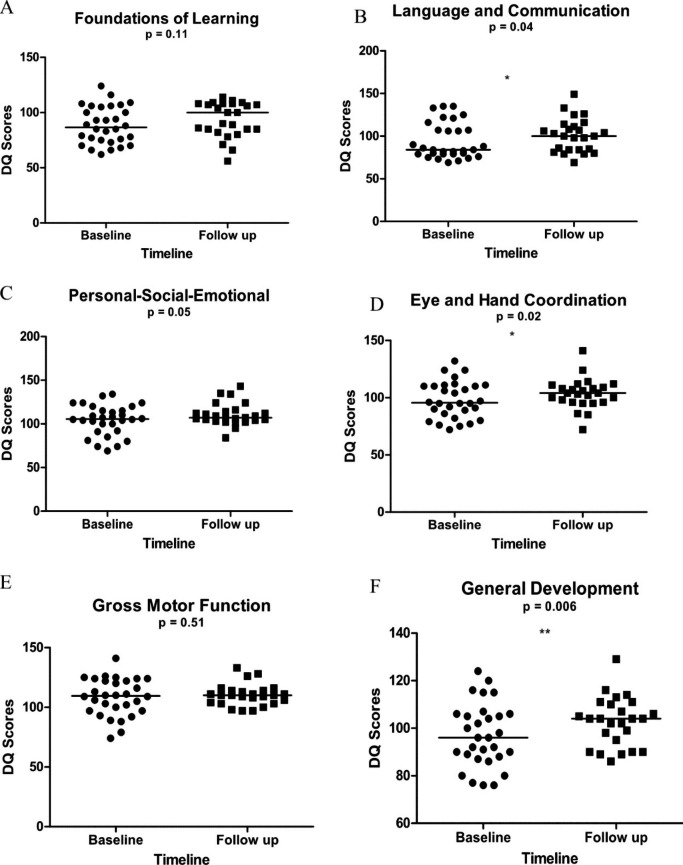
Griffiths Cognitive Developmental Quotients mean scores at baseline and 6 months post treatment follow up timeline
There was a significant improvement in the PSAC DQ medians (Fig. 1) in the Language and Communication Domain (p = 0.003), Eye-Hand Coordination Domain (p = 0.02) and General Development Domain (p = 0.006) after 6 months post treatment. There was also a non-significant improvement in the cognitive domains; Foundations of Learning, Personal Social-Emotional and Gross Motor Function (p > 0.05).
Fig. 1.
(A-F ) Comparisons of the Griffiths Cognitive Developmental Quotients median scores at baseline and 6 months post treatment of pre-school aged children (PSAC) infected with Schistosoma haematobium (n = 25) using the Wilcoxon matched-pairs signed rank test. A = Foundations of Learning, B = Language and Communication, C = Personal-Social-Emotional, D = Eye and Hand Coordination, E = Gross Motor Function and F = General Development
Discussion
Various studies have reported on the prevalence of schistosome infections on PSAC, yet the impact on early childhood development has not yet been realized and quantified [25–28]. This study provides evidence base using a Griffith tool of the negative neuro-developmental association of S. haematobium infections in PSAC in the Foundations of Learning, Eye and Hand Coordination, Language and Communication, Personal-Social-Emotional and Overall General Development Domain after controlling for gender, age, and nutritional status and hemoglobin levels. The associations between S. haematobium infection and the Gross Motor Function were not significant. As this is the first study to our knowledge, reporting on the possible association of S. haematobium infections with early childhood development, we compared our results with previous cognition related research that have been done on children above the age of 5 years. Our findings in the Foundations of Learning Domain and Language and Communication Domain are comparable to results from previous studies that reported associations between schistosome infections and poor performance in both the attention, memory and verbal domains in children older than 5 years [29–34].
We hypothesized that poor cognitive performance in the learning and memory domain might be caused by the inflammatory responses against the schistosome worms and or eggs. Both acute and chronic schistosomiasis cause inflammatory responses that are characterized by the production of pro -inflammatory cytokines such as Interferon gamma (IFN-γ), Interleukin-6 (IL-6), Tumor Necrosis Factor (TNF), and C-reactive protein [34–36], [35–37]. These peripheral inflammatory mediators have been reported to be associated with cognitive decline and impairment as they have been reported to be able to affect the brain via different mechanisms and reported to affect working memory [38–41]. The exact mechanism of how inflammation causes cognitive decline and impairment is unknown and there is need for further research on the mechanistic role of inflammation during urinary schistosomiasis on cognitive functions in addition to the characterization of the inflammatory responses and cognitive responses of populations with schistosome infections.
Multiple confounding factors affect cognitive function [42–46], and controlling for all possible confounding factors is challenging in a field-based study which was one of the limitations of this study. We however factored in the most common and important confounders known to influence cognitive function in PSAC such as age, gender and hemoglobin levels (8, 26). In this study, the gender of PSAC had significant associations in the Eye-Hand Coordination Domain where female PSAC performed better than the male PSAC. Our observations are similar to studies which reported that girls in their early childhood stage usually perform better than the boys in different cognitive tests [47, 48]. Contrary to studies reporting the associations of nutritional status and delayed childhood development, socio-emotional, motor and cognitive development [49–56], we did not find any significant association between nutritional status and performance in any of the cognitive domains. In addition, our study did not show significant association of stunting in PSAC with S. haematobium infections contrary to studies that were conducted in another district (Shamva) in Zimbabwe [28, 37].
We also report an improvement in the Language and Communication Domain, Eye-Hand Coordination Domain (p = 0.02) and General Development Domain (p = 0.006) after 6 months post treatment emphasizing the importance and positive impact of treatment of schistosomiasis in PSAC. Our results are in agreement to those from a study that was done by Nokes et al. on Chinese primary school children who had S. japonicum infections [29].
Study Limitations
Although the study had an increased ratio of uninfected (controls) to infected (cases) PSAC, we acknowledge the low probability of the presence of undetected schistosome infections in some of the PSAC who were classified as controls. We recommend future studies where larger sample sizes are used to include the use of molecular techniques for the diagnosis of infection parallel to the standard diagnostic techniques used for schistosomiasis.
Conclusion
Results of this study provides the evidence base of the possible effects of S. haematobium infections on the neurodevelopment of PSAC using the comprehensive Griffith Mental Developmental Scales III tool under field conditions. We report significant associations between S. haematobium infections and poor performance in the Foundations of Learning, Language and Communication, Personal-Social-Emotional, Eye and Hand Coordination and the Overall General Development Domain in PSAC. Associations between S. haematobium infections and the Gross Motor Function Domain were insignificant. Based on the evidence from the study, we recommend that schistosomiasis prevention and control programs consider inclusion of PSAC in the yearly mass drug treatment programs. This will provide cognitive remediation to PSAC residing in schistosomiasis endemic areas. Furthermore, we recommend development and validation of appropriate tools to stimulate foundations of learning for children in resource-limited settings which are endemic for schistosomiasis together with regular treatment of other parasitic infections.
Acknowledgements
We would like to acknowledge the Ministry of Health and Child Care, the Medical Research Council of Zimbabwe, Village Health Workers, nursing staff, parents and children from Murewa district for their various roles in this work. A special thanks to the psychologist team members and the Biochemistry Department parasitology team at the University of Zimbabwe for technical support during field sampling and parasitological analysis. Our most profound gratitude to the participants and their parents or guardians for taking part in this study.
Abbreviations
- DQ
Developmental Quotient
- EHCDQ
Eye and Hand Coordination Development Quotient
- FLDQ
Foundations of Leaning Development Quotient
- GDDQ
General Development Quotient
- GMDQ
Gross Motor Function Development Quotient
- IFN –γ
Interferon gamma
- IL-6
Interleukin-6
- IQR
Interquartile range
- MDA
Mass Drug Administration
- MRCZ
Medical Research Council of Zimbabwe
- PSAC
Preschool aged children
- PSEDQ
Personal-Social-Emotional Development Quotient
- PZQ
Praziquantel
- S. haematobium
Schistosoma haematobium
- TNF
Tumor Necrosis Factor
- WHO
World Health Organization
Authors’ contributions
MK, SM, FM, DC and TM conceived and designed the study. MK, TMJ, AV, LJ, AM, EM, BDM and HM performed the clinical examination and/or parasitology. RBM and MK analyzed the data. MK wrote the manuscript. All authors read and approved the manuscript.
Funding
The British Academy (BA) supported the field and laboratory activities. The funder was not involved in the design of the study, collection, analysis and interpretation of data, and in writing the manuscript. This research was commissioned by the National Institute of Health Research (NIHR), Global Health Research Programme (16/136/33) using UK aid from UK Government. The views expressed in this publication are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
Ethical approval was obtained from Medical Research Council of Zimbabwe (MRCZ/A/2246 and MRCZ/A/2573). Gatekeeper approval was obtained from the Provincial and District Medical Directors and Community Leaders. Written informed consent was obtained from the parents or guardians of the children. All methods were performed in accordance with relevant guidelines and regulations.
Consent for publication
None required.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Maritha Kasambala, Email: marithakasambala@gmail.com.
Takafira Mduluza, Email: mduluza@medic.uz.ac.zw, Email: tmduluza@yahoo.com.
Arthur Vengesai, Email: arthurvengesai@gmail.com.
Luxwell Jokonya, Email: tljokonya@gmail.com.
Herald Midzi, Email: midziherald@gmail.com.
Rutendo Birri Makota, Email: doclux@gmail.com, Email: rutendobbirri@gmail.com.
Arnold Mutemeri, Email: tinashearnold@gmail.com.
Emmanuel Maziti, Email: emaziti@gzu.ac.zw.
Bazondlile Dube-Marimbe, Email: bazoedube@gmail.com.
Dixon Chibanda, Email: dichi@zol.co.zw.
Francisca Mutapi, Email: f.mutapi@ed.ac.uk.
Samson Mukaratirwa, Email: Mukaratirwa@ukzn.ac.za, Email: SMukaratirwa@rossvet.edu.kn.
References
- 1.Nelwan ML. Schistosomiasis: life cycle, diagnosis, and control. Curr Therapeutic Res. 2019;91:5–9. doi: 10.1016/j.curtheres.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antwi S, Aboah K, Saprong C. The unacknowledged impact of urinary schistosomiasis in children: 5 cases from Kumasi, Ghana. Ghana Med J. 2014;48(4):228–33. doi: 10.4314/gmj.v48i4.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schafer TW, Hale BR. Gastrointestinal complications of schistosomiasis. Curr Gastroenterol Rep. 2001;3(4):293–303. doi: 10.1007/s11894-001-0052-1. [DOI] [PubMed] [Google Scholar]
- 4.de Cleva R, Herman P, Pugliese V, Zilberstein B, Saad WA, Gama-Rodrigues JJ. Fathal pulmonary hypertension after distal splenorenal shunt in schistosomal portal hypertension. World J Gastroenterology: WJG. 2004;10(12):1836. doi: 10.3748/wjg.v10.i12.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y, Ross AG, Hou X, Lou Z, McManus DP. Oriental schistosomiasis with neurological complications: case report. Ann Clin Microbiol Antimicrob. 2011;10(1):1–5. doi: 10.1186/1476-0711-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Development PfC Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Tropical Med Int Health. 2002;7(2):104–17. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- 7.Rujeni N, Nausch N, Midzi N, Cowan GJ, Burchmore R, Cavanagh DR, et al. Immunological consequences of antihelminthic treatment in preschool children exposed to urogenital schistosome infection. Journal of Tropical Medicine. 2013;2013. [DOI] [PMC free article] [PubMed]
- 8.Ezeamama AE, Bustinduy AL, Nkwata AK, Martinez L, Pabalan N, Boivin MJ, et al. Cognitive deficits and educational loss in children with schistosome infection—a systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(1):e0005524. doi: 10.1371/journal.pntd.0005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bundy D. School health and nutrition: policy and programs. FoodNutr Bull. 2005;26(2_suppl2):186-S92. doi: 10.1177/15648265050262S209. [DOI] [PubMed] [Google Scholar]
- 10.Hotez P, Ottesen E, Fenwick A, Molyneux D. The neglected tropical diseases: the ancient afflictions of stigma and poverty and the prospects for their control and elimination. Hot topics in infection and immunity in children III. 2006:23–33. [DOI] [PubMed]
- 11.Wami WM, Nausch N, Midzi N, Gwisai R, Mduluza T, Woolhouse ME, et al. Comparative assessment of health benefits of praziquantel treatment of urogenital schistosomiasis in preschool and primary school-aged children. BioMed research international. 2016;2016. [DOI] [PMC free article] [PubMed]
- 12.Organization WH. Report of a meeting to review the results of studies on the treatment of schistosomiasis in preschool-age children. 2011.
- 13.Osakunor DN, Woolhouse ME, Mutapi F. Paediatric schistosomiasis: What we know and what we need to know. PLoS Negl Trop Dis. 2018;12(2):e0006144. doi: 10.1371/journal.pntd.0006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coulibaly JT, Panic G, Silué KD, Kovač J, Hattendorf J, Keiser J. Efficacy and safety of praziquantel in preschool-aged and school-aged children infected with Schistosoma mansoni: a randomised controlled, parallel-group, dose-ranging, phase 2 trial. The Lancet Global Health. 2017;5(7):e688-e98. doi: 10.1016/S2214-109X(17)30187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pabalan N, Singian E, Tabangay L, Jarjanazi H, Boivin MJ, Ezeamama AE. Soil-transmitted helminth infection, loss of education and cognitive impairment in school-aged children: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12(1):e0005523. doi: 10.1371/journal.pntd.0005523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Learning CCo. State of Learning in Canada: A Year in Review, 2009–2010. Executive Summary. (2010, January 1). The Free library (2010) Retrieved June 04, 2022 from https://wwwthefreelibrarycom/StateofLearninginCanada:AYearinReview,2009-2010Executive-a0276752739.
- 17.Hennessy E, Gormley S, Lopez-Rodriguez AB, Murray C, Murray C, Cunningham C. Systemic TNF-α produces acute cognitive dysfunction and exaggerated sickness behavior when superimposed upon progressive neurodegeneration. Brain Behav Immun. 2017;59:233–44. doi: 10.1016/j.bbi.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vitkovic L, Konsman J, Bockaert J, Dantzer R, Homburger V, Jacque C. Cytokine signals propagate through the brain. Mol Psychiatry. 2000;5(6):604–15. doi: 10.1038/sj.mp.4000813. [DOI] [PubMed] [Google Scholar]
- 19.Miller AH. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav Immun. 2009;23(2):149–58. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–5. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 21.Mott K, Baltes R, Bambagha J, Baldassini B. Field studies of a reusable polyamide filter for detection of Schistosoma haematobium eggs by urine filtration. Tropenmedizin und Parasitologie. 1982;33(4):227–8. [PubMed] [Google Scholar]
- 22.Katz N, Chaves A, Pellegrino J. A simple, device for quantita tive stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400. [PubMed] [Google Scholar]
- 23.Laughton B, Springer P, Grove D, Seedat S, Cornell M, Kidd M, et al. Longitudinal developmental profile of children from low socio-economic circumstances in Cape Town, using the 1996 Griffiths Mental Development Scales. South Afr J Child Health. 2010;4(4):106–11. [PMC free article] [PubMed] [Google Scholar]
- 24.Jacklin L, Cockcroft K. The griffiths mental developmental scales: An overview and a consideration of their relevance for South Africa. Psychological assessment in South Africa: Research and applications. 2013:169 – 85.
- 25.Sacolo-Gwebu H, Chimbari M, Kalinda C. Prevalence and risk factors of schistosomiasis and soil-transmitted helminthiases among preschool aged children (1–5 years) in rural KwaZulu-Natal, South Africa: a cross-sectional study. Infect Dis poverty. 2019;8(1):1–12. doi: 10.1186/s40249-019-0561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Betson M, Sousa-Figueiredo JC, Rowell C, Kabatereine NB, Stothard JR. Intestinal schistosomiasis in mothers and young children in Uganda: investigation of field-applicable markers of bowel morbidity. Am J Trop Med Hyg. 2010;83(5):1048. doi: 10.4269/ajtmh.2010.10-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekpo UF, Oluwole AS, Abe EM, Etta HE, Olamiju F, Mafiana CF. Schistosomiasis in infants and pre-school-aged children in sub-Saharan Africa: implication for control. Parasitology. 2012;139(7):835–41. doi: 10.1017/S0031182012000029. [DOI] [PubMed] [Google Scholar]
- 28.Mduluza-Jokonya TL, Naicker T, Kasambala M, Jokonya L, Vengesai A, Midzi H, et al. Clinical morbidity associated with Schistosoma haematobium infection in pre‐school age children from an endemic district in Zimbabwe. Tropical Med Int Health. 2020;25(9):1110–21. doi: 10.1111/tmi.13451. [DOI] [PubMed] [Google Scholar]
- 29.Nokes C, Grantham-McGregor S, Sawyer A, Cooper E, Bundy D. Parasitic helminth infection and cognitive function in school children. Proceedings of the Royal Society of London Series B: Biological Sciences. 1992;247(1319):77–81. [DOI] [PubMed]
- 30.De Clercq D, Sacko M, Behnke J, Gilbert F, Vercruysse J. The relationship between Schistosoma haematobium infection and school performance and attendance in Bamako, Mali. Annals of Tropical Medicine & Parasitology. 1998;92(8):851–8. doi: 10.1080/00034983.1998.11813350. [DOI] [PubMed] [Google Scholar]
- 31.Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, Bundy D, et al. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hyg. 1999;60(4):556–65. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]
- 32.Sternberg RJ, Powell C, McGrane P, Grantham-McGregor S. Effects of a parasitic infection on cognitive functioning. J Experimental Psychology: Appl. 1997;3(1):67. [Google Scholar]
- 33.Ezeamama AE, Friedman JF, Acosta LP, Bellinger DC, Langdon GC, Manalo DL, et al. Helminth infection and cognitive impairment among Filipino children. Am J Trop Med Hyg. 2005;72(5):540. doi: 10.4269/ajtmh.2005.72.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musuva R, Shen Y, Wei X, Binder S, Ivy JA, Secor WE, et al. Change in children’s school behavior after mass administration of praziquantel for Schistosoma mansoni infection in endemic areas of western Kenya: A pilot study using the Behavioral Assessment System for Children (BASC-2) PLoS ONE. 2017;12(7):e0181975. doi: 10.1371/journal.pone.0181975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huot C, Clerissi C, Gourbal B, Galinier R, Duval D, Toulza E. Schistosomiasis vector snails and their microbiota display a phylosymbiosis pattern. Front Microbiol. 2020;10:3092. doi: 10.3389/fmicb.2019.03092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pearce EJ, MacDonald AS. The immunobiology of schistosomiasis. Nat Rev Immunol. 2002;2(7):499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 37.Chimponda TN, Mushayi C, Osakunor DN, Vengesai A, Enwono E, Amanfo S, et al. Elevation of C-reactive protein, P-selectin and Resistin as potential inflammatory biomarkers of urogenital Schistosomiasis exposure in preschool children. BMC Infect Dis. 2019;19(1):1–8. doi: 10.1186/s12879-019-4690-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1β have different effects on memory consolidation. Brain Res Bull. 2005;67(1–2):24–9. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 39.Seguin JA, Brennan J, Mangano E, Hayley S. Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatr Dis Treat. 2009;5:5. [PMC free article] [PubMed] [Google Scholar]
- 40.Liu X, Wu Z, Hayashi Y, Nakanishi H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience. 2012;216:133–42. doi: 10.1016/j.neuroscience.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 41.Guo G, Harris KM. The mechanisms mediating the effects of poverty on children’s intellectual development. Demography. 2000;37(4):431–47. doi: 10.1353/dem.2000.0005. [DOI] [PubMed] [Google Scholar]
- 42.Bradley RH, Corwyn RF. Socioeconomic status and child development. Ann Rev Psychol. 2002;53(1):371–99. doi: 10.1146/annurev.psych.53.100901.135233. [DOI] [PubMed] [Google Scholar]
- 43.Jefferis BJ, Power C, Hertzman C. Birth weight, childhood socioeconomic environment, and cognitive development in the 1958 British birth cohort study. BMJ. 2002;325(7359):305. doi: 10.1136/bmj.325.7359.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santos DN, Assis AMO, Bastos ACS, Santos LM, Santos CAS, Strina A, et al. Determinants of cognitive function in childhood: a cohort study in a middle income context. BMC Public Health. 2008;8(1):1–15. doi: 10.1186/1471-2458-8-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeung W-JJ, Pfeiffer KM. The Black–White test score gap and early home environment. Soc Sci Res. 2009;38(2):412–37. doi: 10.1016/j.ssresearch.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 46.Christensen DL, Schieve LA, Devine O, Drews-Botsch C. Socioeconomic status, child enrichment factors, and cognitive performance among preschool-age children: results from the Follow-Up of Growth and Development Experiences study. Res Dev Disabil. 2014;35(7):1789–801. doi: 10.1016/j.ridd.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Palejwala MH, Fine JG. Gender differences in latent cognitive abilities in children aged 2 to 7. Intelligence. 2015;48:96–108. doi: 10.1016/j.intell.2014.11.004. [DOI] [Google Scholar]
- 48.Von Stumm S, Plomin R. Socioeconomic status and the growth of intelligence from infancy through adolescence. Intelligence. 2015;48:30–6. doi: 10.1016/j.intell.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B, et al. Developmental potential in the first 5 years for children in developing countries. The lancet. 2007;369(9555):60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martorell R, Horta BL, Adair LS, Stein AD, Richter L, Fall C, Consortium on Health Oriented Research in Transitional Societies Group et al. Weight Gain in the First Two Years of Life Is an Important Predictor of Schooling Outcomes in Pooled Analyses from Five Birth Cohorth from Low and Midle Income Countries. J Nutr. 2010;140:348–54. doi: 10.3945/jn.109.112300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yousafzai AK, Rasheed MA, Bhutta ZA. Annual research review: improved nutrition–a pathway to resilience. J Child Psychol Psychiatry. 2013;54(4):367–77. doi: 10.1111/jcpp.12019. [DOI] [PubMed] [Google Scholar]
- 52.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet (London England) 2013;382(9890):427–51. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 53.Stephenson LS, Latham MC, Kurz KM, Kinoti SN. Single dose metrifonate or praziquantel treatment in Kenyan children. II. Effects on growth in relation to Schistosoma haematobium and hookworm egg counts. Am J Trop Med Hyg. 1989;41(4):445–53. doi: 10.4269/ajtmh.1989.41.445. [DOI] [PubMed] [Google Scholar]
- 54.Assis AdO, Prado MdS, Barreto ML, Reis MGd C, Pinheiro S, Parraga IM, et al. Childhood stunting in Northeast Brazil: the role of Schistosoma mansoni infection and inadequate dietary intake. Eur J Clin Nutr. 2004;58(7):1022–9. doi: 10.1038/sj.ejcn.1601926. [DOI] [PubMed] [Google Scholar]
- 55.Coutinho HM, Acosta LP, McGarvey ST, Jarilla B, Jiz M, Pablo A, et al. Nutritional status improves after treatment of schistosoma japonicum–infected children and adolescents. J Nutr. 2006;136(1):183–8. doi: 10.1093/jn/136.1.183. [DOI] [PubMed] [Google Scholar]
- 56.Choto ET, Mduluza T, Mutapi F, Chimbari MJ. Association of schistosomiasis and risk of prostate cancer development in residents of Murehwa rural community, Zimbabwe. Infect Agents Cancer. 2020;15(1):1–11. doi: 10.1186/s13027-020-00327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.