Abstract
Background
Understanding how booster vaccination can prevent moderate and severe illness without hospitalization is crucial to evaluate the full advantage of mRNA boosters.
Methods
We followed 85 801 participants (aged 31–81 years) in 2 large population-based cohorts during the Omicron BA.1/2 wave. Information on home testing, PCR testing, and symptoms of coronavirus disease 2019 (COVID-19) was extracted from biweekly questionnaires covering the period 12 January 2022 to 7 April 2022. Vaccination status and data on previous SARS-CoV-2 infection were obtained from national registries. Cox regression was used to estimate the effectiveness of booster vaccination compared to receipt of 2-dose primary series >130 days previously.
Results
The effectiveness of booster vaccination increased with increasing severity of COVID-19 and decreased with time since booster vaccination. The effectiveness against severe COVID-19 was reduced from 80.9% shortly after booster vaccination to 63.4% in the period >90 days after vaccination. There was hardly any effect against mild COVID-19. The effectiveness tended to be lower among subjects aged ≥60 years than those aged <50 years.
Conclusions
This is the first population-based study to evaluate booster effectiveness against self-reported mild, moderate, and severe COVID-19. Our findings contribute valuable information on duration of protection and thus timing of additional booster vaccinations.
Keywords: SARS-CoV-2, COVID-19, booster vaccination, disease severity, mRNA vaccine, MoBa, vaccine effectiveness
Understanding how booster vaccination can prevent moderate and severe illness without hospitalization is crucial to evaluate the full advantage of mRNA boosters. In this population-based study, mRNA booster vaccination partly prevented moderate and severe COVID-19, but not mild COVID-19.
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant B.1.1.529 (Omicron) emerged in November 2021 in South Africa and was declared a variant of concern by the World Health Organization on 26 November 2021 [1]. In Norway, the first case of Omicron was detected after a Christmas party held on 26 November [2, 3]. By late December, Omicron BA.1 was the dominant strain in Norway [4]. In the second week of January 2022, Omicron was detected in 94% of all sequenced cases [5]. BA.1 was then gradually replaced by BA.2. By the middle of March, BA.2 was the dominant strain [6].
Vaccines against SARS-CoV-2 have proven efficacious against SARS-CoV-2 infection and coronavirus disease (COVID-19) in both clinical trials and observational real-world studies [7, 8]. However, the effectiveness after the primary course with 2 doses wanes over time, especially among elderly subjects and people with underlying diseases [7, 9–11]. Moreover, the effectiveness has been reported to be lower against the Omicron than against the Delta variant [10, 12–16]. Omicron is associated with less-severe disease compared to earlier SARS-CoV-2 variants of concern [17]. Nevertheless, severe disease is frequently observed among unvaccinated, elderly Omicron cases [18].
Previous studies have reported vaccine effectiveness against infection and/or hospitalization [10, 12, 16]. There is, however, a need to understand effectiveness across the whole spectrum of disease severity. Most COVID-19 cases do not require medical care, and hospitalization rates therefore poorly reflect the total disease burden in the population. We have followed a large, population-based cohort with detailed information on testing and symptoms. Our aim was to estimate effectiveness of booster vaccination against SARS-CoV-2 on mild, moderate, and severe COVID-19, and to describe the duration of protection.
METHODS
In Norway, the national immunization program against SARS-CoV-2 started on 27 December 2020 [11]. In the primary 2-dose vaccine course, most subjects received the mRNA vaccines Comirnaty (Pfizer/BioNTech; BNT162b2), or Spikevax (Moderna; mRNA-1273), or a combination of the 2. The adenovector-based vaccine Vaxzevria (AstraZeneca; ChAdOx nCoV-19; AZD1222) was only offered through the program until March 2021 [19].
Booster vaccination was recommended from 5 October 2021 to risk groups and people 65 years and older, then to all adults 45 years and older from 26 November 2021. Although not issued as a recommendation, adults aged 18–45 years were also eligible for booster vaccination. Only mRNA vaccines were offered as booster. In December, the minimum interval between dose 2 and the booster dose was reduced from 6 months to 20 weeks [20]. By January 2022, 88% of subjects 18 years and older had received at least 2 vaccine doses, while 51.2% were vaccinated with 3 doses [5]. Among adults 45 years and older, the main target group for booster vaccination, the coverage of 3 doses was 73.3%.
Surveillance of SARS-CoV-2 infections in Norway was initially based on mandatory reporting of laboratory-confirmed (polymerase chain reaction [PCR]) infections to the Norwegian Surveillance System for Communicable Disease (MSIS). The high transmission rate of the Omicron variant, limited laboratory capacity, and increased availability of SARS-CoV-2 antigen self-testing kits led the government to recommend self-testing from 12 February 2022 [21]. As a consequence, there has been an increased underreporting of SARS-CoV-2 cases to MSIS. MSIS can therefore no longer be used to assess vaccine effectiveness, as previously done in Norway [11, 12, 22]. Thus, population-based cohorts with frequent follow-up of self-testing, PCR testing, and symptoms are needed for estimates of vaccine effectiveness.
The Norwegian Mother, Father, and Child Cohort Study and the Senior Cohort
The Norwegian Mother, Father, and Child Cohort Study (MoBa) is a population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health. Participants were recruited from all over Norway during 1999–2008 [23]. The women consented to participation in 41% of the pregnancies. Since March 2020, adult participants have been invited every 14 days to answer electronic questionnaires on the SARS-CoV-2 pandemic. We used data from 5 consecutive questionnaires (Q1–Q5) sent out to 101 765 participants in January-March, 2022. The response rates were 67%–72%.
To cover older age groups, a senior cohort was established in December 2020. About 13 000 randomly selected adults, aged 65–80 years living in Oslo, were invited, and 36% consented to participation. Four questionnaires (Q1–Q4), distributed to 4804 participants in January-March 2022, have been included. The response rates were 88%–95%.
The participants in the 2 cohorts were asked identical questions about testing for SARS-CoV-2, symptoms of COVID-19, and severity of symptomatic COVID-19. The participants were not given instructions on test frequency or indication. Each questionnaire was open for response for 14 days.
The study was approved by The Regional Committee for Medical and Health Research Ethics, Southeast Norway. Written informed consent was obtained from all participants.
Exposure
Information on vaccinations was obtained from the Norwegian Immunization Registry. Notification of vaccination against COVID-19 is mandatory. Booster vaccination was defined as a third vaccine dose received at least 130 days after dose 2. According to national recommendations, the minimum interval between the second and third doses should be 20 weeks (140 days). However, we used 130 days as a cutoff because many received the third dose a few days early. In the study population, the vaccination coverage of at least 1 dose was very high (98%), thus the unvaccinated was not a suitable reference group. We therefore compared booster vaccination to the primary series of 2 doses, estimating relative vaccine effectiveness (rVE) [24].
Outcome
SARS-CoV-2 infection was defined as a self-reported positive test for SARS-CoV-2. The test could be either a self-sample rapid antigen test or a laboratory test (PCR).
COVID-19 was defined as symptomatic SARS-CoV-2 infection. Participants with a SARS-CoV-2 infection were defined as symptomatic if they answered “yes” to the question “have you been feeling ill, had respiratory symptoms, or fever during the last 14 days” in the same questionnaire where they reported the positive test.
The severity of COVID-19 was based on the question “how ill did you feel?” and classified as mild illness (“barely ill”), moderate illness (“moderately ill, bedridden for several days”), or severe illness (“very ill”). The infected participants who did not answer this question or who reported no symptoms were only included among all SARS-CoV-2 infections.
In Q4 and Q5, participants were asked specifically about the date of the positive SARS-CoV-2 test. In Q1–Q3, participants could only indicate whether they had tested positive at any time during the last 14 days. The participants were also asked how many days prior to filling out the questionnaire potential COVID-19 symptoms occurred (0–1, 2–3, 4–5, 6–7, 8–9, or 10–14 days). We estimated the date of infection by sampling randomly among the candidate dates of symptom onset. For participants who reported a positive test but no symptoms, the date of infection was estimated by sampling randomly from all the 14 dates covered in that questionnaire period.
Covariates
Information on sex, age, and county of residence was obtained from the existing MoBa and senior cohort databases. Information from MSIS was used to identify participants with a previous SARS-CoV-2 infection, defined as a registered infection with a date more than 14 days prior to the participant's start of follow-up (defined below).
Follow-Up and Study Sample
In each questionnaire, the participants reported test activity and symptoms during the period from 14 days prior to and up to the fill-in date. Thus, for each questionnaire they returned, the participants could contribute up to 15 days of follow-up time. Participants may have answered the questionnaires at uneven time intervals or not answered all questionnaires and may therefore have questionnaire periods that partly overlap or have gaps between them.
Each participant's start of follow-up was defined as the start date of their first questionnaire period or the date corresponding to 130 days after their second dose, whichever occurred last. The participants were followed until the last day of their last questionnaire period, receipt of a fourth vaccine dose, or the date of the first reported SARS-CoV-2 infection, whichever occurred first. The follow-up fell within the periods 12 January to 7 April 2022, for MoBa participants, and 13 January to 24 March 2022 for the senior cohort participants.
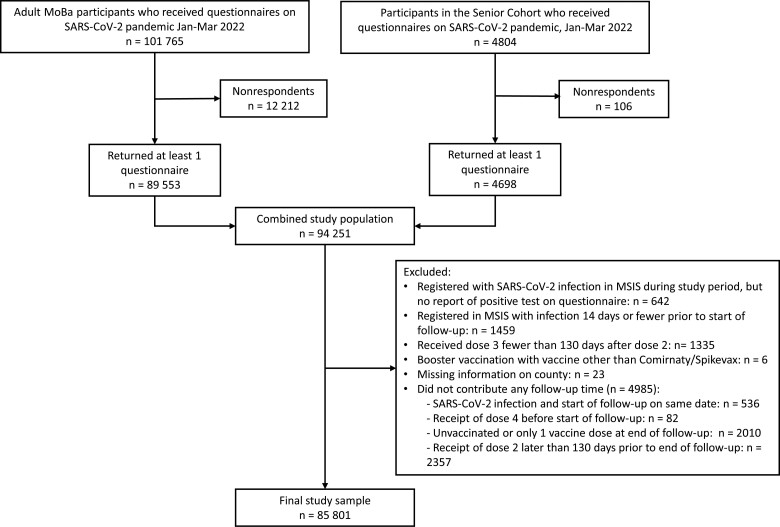
Only participants answering at least 1 of the questionnaires were eligible for inclusion in the analysis (Figure 1). We excluded 642 participants who were registered with a SARS-CoV-2 infection in MSIS during the study period but did not report a positive test for SARS-CoV-2 on any of the questionnaires. We also excluded 1459 participants registered with an infection in MSIS 14 days or fewer prior to start of follow-up, because it was difficult to differentiate between previous infections and reported outcomes in this period. We furthermore excluded 1335 participants who received dose 3 fewer than 130 days after dose 2, 6 participants who received booster vaccines other than Comirnaty or Spikevax, and 23 participants with missing information on county of residence. Finally, we excluded 4985 participants who did not contribute any follow-up time. In total, 85 801 participants were included in the final study sample (Figure 1).
Figure 1.
Flow chart of the study population. Abbreviations: MoBa, Norwegian Mother, Father, and Child Cohort Study; MSIS, Norwegian Surveillance System for Communicable Disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Statistical Analysis
We defined the test frequency for each questionnaire as the number of participants reporting in the questionnaire that they had been tested for SARS-CoV-2 during the last 14 days divided by the number of participants responding to the questionnaire. We calculated test frequency with any test, antigen test, and PCR test (PCR test alone or PCR test plus antigen test). Furthermore, we assessed test frequency by vaccination status at the fill-in date of the questionnaire, sex, and age. We also assessed reasons for testing by vaccination status.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between vaccination and risk of SARS-CoV-2 infection were estimated with Cox regression. We used a stratified Cox model with county as strata and calendar time as the underlying time scale. Vaccination was treated as a time-dependent covariate. The reference category consisted of person-time more than 130 days after receipt of the second vaccine dose up until booster vaccination, that is, person-time during which the participants were eligible for, but had not yet received, booster vaccination. Booster vaccination was categorized based on time since vaccination (0–6, 7–30, 31–60, 61–90, 91–120, and >120 days). Full effect of the booster vaccination is not expected until 7 days after vaccination. The model was adjusted for previous SARS-CoV-2 infection, sex, and age group (<40, 40–49, 50–59, and ≥ 60 years). The rVE of booster vaccination compared to 2 doses was calculated as 100% multiplied by (1 − HR). In addition to SARS CoV-2 infection, we also used the categories of COVID-19 severity as outcomes: mild, moderate, and severe. In these analyses, participants were censored at the time of infection if their infection was not included in the severity outcome in question.
We performed additional analyses limited to participants with no previous SARS-CoV-2 infection. We also examined the effectiveness of each type of booster vaccine. In this analysis, booster vaccination was categorized both according to time since vaccination and type of vaccine (Comirnaty or Spikevax). We also performed analyses by sex and age (<50, 50–59, and ≥60 years). Here, the categories moderate and severe COVID-19 were combined due to few severe cases.
Nonresponse to a questionnaire may be related to the outcome. To assess whether the missingness was informative, we performed a sensitivity analysis including only participants who returned all questionnaires.
RESULTS
The majority of the 85 801 participants were included in MoBa (94.7%), and 61.3% were women (Table 1). The median age was 49 years (range, 30–81 years); most participants (89.6%) were aged 40–59 years. About 5% had been infected with SARS-CoV-2 prior to the start of follow-up. At the start of follow-up, 23 297 participants (27.2%) had only received 2 vaccine doses, but more than half of these (13 813 participants, 59.3%), received the booster during follow-up. The median follow-up time was 40 days (range, 1–74 days).
Table 1.
Characteristics at Start of Follow-up of the Participants in the Norwegian Mother, Father, and Child Cohort Study (MoBa) and Senior Cohort Included in Vaccine Effectiveness Analyses, N = 85 801
| Characteristic | Value |
| MoBa | 81 290 (94.7) |
| Senior cohort | 4511 (5.3) |
| Sex, women | 52 638 (61.3) |
| Returned all questionnaires, yesa | 48 379 (56.4) |
| Age groups, y | |
| <40 | 2082 (2.4) |
| 40–49 | 41 867 (48.8) |
| 50–59 | 34 999 (40.8) |
| 60–69 | 3639 (4.2) |
| ≥70 | 3214 (3.7) |
| Previous COVID-19 infection, yesb | 3928 (4.6) |
| COVID-19 vaccination status at start of follow-upc | |
| Dose 1 | |
| Date dose 1, median | 17 June 2021 |
| Interval in days between dose 1 and 2, median | 47 |
| Comirnaty | 68 750 (80.1) |
| Spikevax | 11 666 (13.6) |
| Vaxzevria or other | 5385 (6.3) |
| Dose 2 | |
| Comirnaty | 62 068 (72.3) |
| Spikevax | 23 715 (27.6) |
| Vaxzevria or other | 18 (0.02) |
| Booster dose | |
| Comirnaty | 39 251 (45.7) |
| Spikevax | 23 253 (27.1) |
| No booster dose before start of follow-up | 23 297 (27.2) |
Data are No. (%) except where indicated.
Participants in MoBa received 5 questionnaires and participants in the senior cohort received 4 questionnaires.
Defined as an infection registered in the Norwegian Surveillance System for Communicable Disease with date more than 14 days prior to the participant's start of follow-up.
All 85 801 participants included in the analyses had received at least 2 vaccine doses at start of follow-up.
Of the participants, 75 911 (88.5%) reported to have been tested for SARS-CoV-2 during the last 14 days on at least 1 questionnaire. Test frequency was highest in Q2 (65.2%) and lowest in Q5 (25.3%) (Supplementary Figure 1). The antigen test frequency was much higher than the PCR test frequency, which decreased from 12.5% (Q1) to 2.2% (Q5) (Supplementary Figure 2). Test frequency was higher among booster-vaccinated participants than among participants with 2 doses, that is, those who had not yet received the booster (Supplementary Figure 3). However, participants with 2 doses were more likely to have taken a PCR test (Supplementary Figure 4). Test frequency was higher among women than among men and decreased with age (Supplementary Figures 5 and 6). Having symptoms was a more common reason for testing among those without a booster (Supplementary Figure 7).
In total, 41 462 participants (48.3%) were infected with SARS-CoV-2 during follow-up. Of those infected, 18 519 (44.7%), 20 440 (49.3%), and 1247 (3.0%) reported mild, moderate, and severe COVID-19, respectively. The remaining 1256 cases (3.0%) were either asymptomatic (n = 1102) or not possible to classify (n = 154) because they had not answered the relevant questions. Only 3 of the severe COVID-19 cases reported to have been hospitalized during the same period.
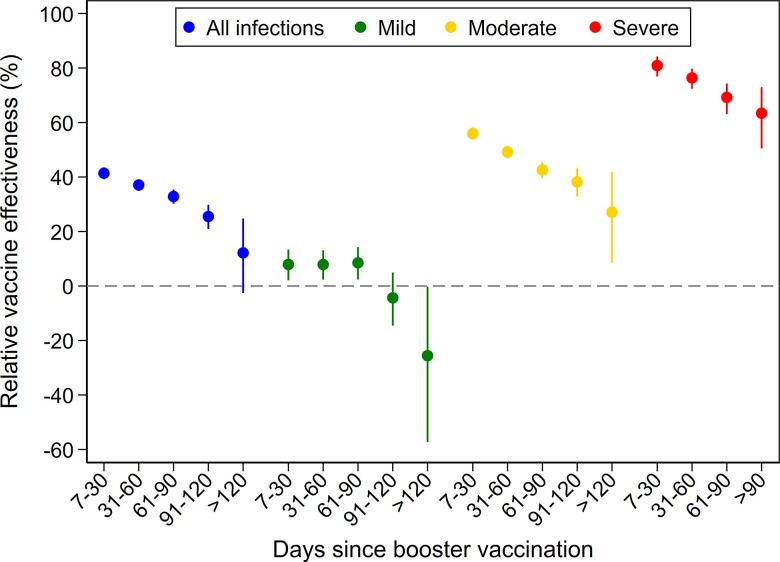
The rVE of the booster vaccination increased with increasing severity of symptoms, while it decreased with time since the booster (Table 2 and Figure 2). For SARS-CoV-2 infections, regardless of symptoms, rVE was 41.4% (95% CI, 39.2%–43.5%) 7–30 days after booster vaccination and decreased to 12.2% (95% CI, −2.6% to 24.8%) > 120 days after vaccination. The rVE against mild illness was only 7.9% (95% CI, 2.1%–13.4%) shortly after vaccination and negative >120 days after vaccination. For moderate illness, rVE was 56.0% (95% CI, 53.7%–58.1%) and 27.1% (95% CI, 8.6%–41.8%), respectively, in the 2 time periods. For severe disease, rVE decreased from 80.9% (95% CI, 76.9%–84.2%) 7–30 days after vaccination to 63.4% (95% CI, 50.5%–72.9%) >90 days after vaccination.
Table 2.
Effectiveness of Booster Vaccination with mRNA Vaccine Against SARS-CoV-2 Infection and COVID-19 Caused by the Omicron Variant Among Participants in the Norwegian Mother, Father, and Child Cohort Study and the Senior Cohort, N = 85 801
| SARS-CoV-2 Infection | Mild COVID-19 | Moderate COVID-19 | Severe COVID-19 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Interval From Vaccination, d | Person-Time, d | Cases, na | rVEb (95% CI) | Cases, n | rVEb (95% CI) | Cases, n | rVEb (95% CI) | Cases, n | rVEb (95% CI) |
| 2 dosesc | |||||||||
| >130 | 419 536 | 4895 | Ref | 1607 | Ref | 2858 | Ref | 307 | Ref |
| Booster vaccinationd | |||||||||
| 0–6 | 115 285 | 864 | 32.4 (27.2–37.2) | 412 | 0.9 (−10.8 to 11.3) | 396 | 45.6 (39.5–51.2) | 28 | 61.9 (43.5–74.3) |
| 7–30 | 864 567 | 8506 | 41.4 (39.2–43.5) | 4177 | 7.9 (2.1–13.4) | 3819 | 56.0 (53.7–58.1) | 179 | 80.9 (76.9–84.2) |
| 31–60 | 1 168 176 | 16 324 | 37.1 (34.9–39.2) | 7506 | 7.9 (2.3–13.1) | 7912 | 49.2 (46.8–51.4) | 408 | 76.4 (72.4–79.8) |
| 61–90 | 677 064 | 8877 | 32.8 (30.2–35.4) | 3875 | 8.5 (2.4–14.2) | 4512 | 42.6 (39.5–45.5) | 264 | 69.2 (63.1–74.3) |
| 91–120 | 171 546 | 1826 | 25.5 (20.9–29.8) | 859 | −4.4 (−14.5 to 4.9) | 864 | 38.2 (32.9–43.2) | 61 | 63.4 (50.5–72.9)e |
| >120 | 13 763 | 170 | 12.2 (−2.6 to 24.8) | 83 | −25.6 (−57.3 to −.3) | 79 | 27.1 (8.6–41.8) | …e | …e |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; Ref, reference; rVE, relative vaccine effectiveness; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Includes asymptomatic SARS-CoV-2 infections and SARS-CoV-2 infection not possible to classify according to severity. Thus, the number of cases exceeds the sum of mild, moderate, and severe COVID-19 cases.
rVE = 100% × (1 − HR). HR was estimated using a stratified Cox model with county as strata and calendar time as the underlying time scale. The model was adjusted for previous SARS-CoV-2 infection, age, and sex.
The reference category consisted of person-time more than 130 days after the second vaccine dose up until booster vaccination.
Booster vaccination was defined as a third vaccine dose received at least 130 days after the second dose.
For severe COVID-19, the upper category was receipt of booster vaccination more than 90 days previously.
Figure 2.
Relative effectiveness of booster vaccination (third dose) with mRNA vaccine against SARS-CoV-2 infection caused by the Omicron variant compared to a reference group vaccinated with 2 doses. Bars represent 95% confidence intervals of the relative vaccine effectiveness.
When we excluded participants with a previous SARS-CoV-2 infection, results were basically unchanged for moderate and severe COVID-19. For mild COVID-19, the negative effect observed in the period >120 days after vaccination was somewhat attenuated (Supplementary Table 1).
The effectiveness tended to be higher among men than among women (Table 3). In the period 7–30 days after booster vaccination, the effectiveness against moderate or severe COVID-19 was 62.6% (95% CI, 59.1%–65.8%) among men, as compared to 56.5% (95% CI, 53.9%–59.0%) among women. However, >90 days after vaccination, rVE was slightly higher among women.
Table 3.
Effectiveness of Booster Vaccination With mRNA Vaccine Against SARS-CoV-2 Infection and COVID-19 Caused by the Omicron Variant Among Participants in the Norwegian Mother, Father, and Child Cohort Study and the Senior Cohort, by Sex
| SARS-CoV-2 Infection | Mild COVID-19 | Moderate or Severe COVID-19 | |||||
|---|---|---|---|---|---|---|---|
| Interval From Vaccination, d | Person Time, d | Cases, na | rVEb (95% CI) | Cases, n | rVEb (95% CI) | Cases, n | rVEb (95% CI) |
| Men, n = 33 163 | |||||||
| 2 dosesc | |||||||
| >130 | 153 995 | 1604 | Ref | 669 | Ref | 883 | Ref |
| Booster vaccinationd | |||||||
| 0–6 | 47 266 | 303 | 40.3 (32.3–47.4) | 164 | 20.7 (5.5–33.5) | 124 | 55.4 (46.0–63.2) |
| 7–30 | 358 248 | 3273 | 43.8 (40.2–47.3) | 1876 | 19.9 (12.1–27.0) | 1250 | 62.6 (59.1–65.8) |
| 31–60 | 459 962 | 6107 | 39.5 (35.8–43.0) | 3171 | 22.7 (15.5–29.4) | 2713 | 53.1 (49.1–55.8) |
| 61–90 | 258 004 | 2862 | 35.6 (30.9–39.9) | 1461 | 23.0 (14.5–30.6) | 1304 | 46.2 (40.7–51.2) |
| >90 | 63 762 | 570 | 21.6 (12.5–29.8) | 324 | 3.0 (−13.3 to 16.9) | 232 | 35.2 (23.7–45.0) |
| Women, n = 52 638 | |||||||
| 2 dosesc | |||||||
| >130 | 265 541 | 3291 | Ref | 938 | Ref | 2282 | Ref |
| Booster vaccinationd | |||||||
| 0–6 | 68 019 | 561 | 27.5 (20.6–33.9) | 248 | −15.9 (−33.9 to −.3) | 300 | 43.4 (36.0–49.9) |
| 7–30 | 506 319 | 5233 | 40.2 (37.4–42.8) | 2301 | −0.7 (−9.1 to 7.2) | 2748 | 56.5 (53.9–59.0) |
| 31–60 | 708 214 | 10 217 | 35.9 (33.2–38.5) | 4335 | −3.2 (−11.4 to 4.4) | 5607 | 51.5 (48.9–53.9) |
| 61–90 | 419 060 | 6015 | 31.5 (28.3–34.6) | 2414 | −1.9 (−10.8 to 6.2) | 3472 | 44.7 (41.4–47.8) |
| >90 | 121 547 | 1426 | 25.4 (20.1–30.3) | 618 | −13.0 (−26.6 to −.9) | 772 | 41.1 (35.6–46.1) |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; Ref, reference; rVE, relative vaccine effectiveness; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Includes asymptomatic SARS-CoV-2 infections and SARS-CoV-2 infection not possible to classify according to severity. Thus, the number of cases exceeds the sum of mild, moderate, and severe COVID-19 cases.
rVE = 100% × (1 − HR). HR was estimated using a stratified Cox model with county as strata and calendar time as the underlying time scale. The model was adjusted for prior SARS-CoV-2 infection and age.
The reference category consisted of person-time more than 130 days after the second vaccine dose up until booster vaccination.
Booster vaccination was defined as a third vaccine dose received at least 130 days after the second dose.
During the first 90 days after booster vaccination, the effectiveness was similar in the 2 youngest age groups (<50 years and 50–59 years; Table 4). However, >90 days after vaccination the effectiveness was lower among the 50–59 year olds. The effectiveness tended to be lower among those ≥60 years as compared to those <50 years, but confidence intervals in the ≥60 years category were wide.
Table 4.
Effectiveness of Booster Vaccination With mRNA Vaccine Against SARS-CoV-2 Infection and COVID-19 Caused by the Omicron Variant Among Participants in the Norwegian Mother, Father, and Child Cohort Study and the Senior Cohort, by Age
| SARS-CoV-2 Infection | Mild COVID-19 | Moderate or Severe COVID-19 | |||||
|---|---|---|---|---|---|---|---|
| Interval From Vaccination, d | Person-Time, d | Cases, na | rVEb (95% CI) | Cases, n | rVEb (95% CI) | Cases, n | rVEb (95% CI) |
| Age group < 50 y, n = 43 949 | |||||||
| 2 dosesc | |||||||
| >130 | 256 116 | 3578 | Ref | 1121 | Ref | 2369 | Ref |
| Booster vaccinationd | |||||||
| 0–6 | 68 470 | 629 | 34.5 (28.6–39.9) | 285 | 4.6 (−9.1 to 16.5) | 327 | 47.3 (40.7–53.2) |
| 7–30 | 445 173 | 5727 | 42.2 (39.6–44.6) | 2655 | 8.0 (.9–14.5) | 2848 | 58.2 (55.8–60.5) |
| 31–60 | 522 742 | 9182 | 37.0 (34.3–39.5) | 4011 | 4.2 (−2.9 to 10.8) | 4885 | 52.1 (49.5–54.5) |
| 61–90 | 246 202 | 4403 | 32.3 (29.0–35.5) | 1826 | 1.3 (−7.2 to 9.1) | 2464 | 46.6 (43.2–49.8) |
| >90 | 55 056 | 814 | 32.7 (27.0–38.0) | 343 | 0.9 (−12.9 to 13.0) | 454 | 47.0 (41.0–52.4) |
| Age group 50–59 y, n = 34 999 | |||||||
| 2 dosesc | |||||||
| 130+ | 149 642 | 1251 | Ref | 453 | Ref | 766 | Ref |
| Booster vaccinationd | |||||||
| 0–6 | 45 004 | 231 | 31.9 (21.3–41.1) | 125 | −1.6 (−24.8 to 17.2) | 95 | 52.5 (40.9–61.9) |
| 7–30 | 397 544 | 2712 | 41.6 (37.3–45.6) | 1483 | 9.4 (−1.3 to 19.0) | 1125 | 60.7 (56.7–64.3) |
| 31–60 | 563 523 | 6749 | 35.9 (31.7–39.9) | 3263 | 10.1 (.1 to 19.0) | 3295 | 51.0 (46.7–54.9) |
| 61–90 | 300 131 | 3789 | 33.1 (28.2–37.6) | 1654 | 15.9 (5.8–24.9) | 2044 | 43.6 (38.3–48.5) |
| >90 | 56 633 | 717 | 18.5 (10.0–26.2) | 316 | −3.4 (−20.8 to 11.6) | 378 | 33.4 (24.0–41.7) |
| Age group ≥ 60 y, n = 6853 | |||||||
| 2 dosesc | |||||||
| >130 | 13 778 | 66 | Ref | 33 | Ref | 30 | Ref |
| Booster vaccinationd | |||||||
| 0–6 | 1811 | 4 | 27.9 (−101.0 to 74.2) | 2 | 25.1 (−218.0 to 82.4) | 2 | 8.2 (−294.7 to 78.6) |
| 7–30 | 21 850 | 67 | 34.6 (5.8 to 54.6) | 39 | 24.9 (−23.5 to 54.3) | 25 | 43.3 (−.4 to 68.0) |
| 31–60 | 81 911 | 393 | 20.5 (−4.7 to 39.6) | 232 | 9.1 (−33.5 to 38.1) | 140 | 36.0 (2.7–57.8) |
| 61–90 | 130 731 | 685 | 21.2 (−3.2 to 39.8) | 395 | 8.6 (−33.6 to 37.4) | 268 | 28.6 (−7.1 to 52.4) |
| >90 | 73 620 | 465 | 24.3 (−.9 to 43.3) | 283 | −7.3 (−62.0 to 28.9) | 172 | 37.5 (3.2–59.6) |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; HR, hazard ratio; Ref, reference; rVE, relative vaccine effectiveness; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Includes asymptomatic SARS-CoV-2 infections and SARS-CoV-2 infection not possible to classify according to severity. Thus, the number of cases exceeds the sum of mild, moderate, and severe COVID-19 cases.
rVE = 100% × (1 − HR). HR was estimated using a stratified Cox model with county as strata and calendar time as the underlying time scale. The model was adjusted for prior SARS-CoV-2 infection, age, and sex.
The reference category consisted of person-time more than 130 days after the second vaccine dose up until booster vaccination.
Booster vaccination was defined as a third vaccine dose received at least 130 days after the second dose.
There was no difference in effectiveness between the 2 mRNA vaccine types used for booster vaccination (Supplementary Table 2). Results from analyses limited to the 48 379 participants who returned all questionnaires were similar to the results for the full study sample (Supplementary Table 3).
DISCUSSION
In this large, population-based cohort study with more than 85 000 participants, we found that booster vaccination gave substantially better protection against severe and moderate than mild COVID-19, and that the protection decreased with time since vaccination. Furthermore, the effectiveness tended to be lower among those ≥60 years as compared to the younger age groups. We found no difference in effectiveness between the 2 mRNA vaccines used for booster vaccination in Norway.
In agreement with recent publications [15, 25, 26], we found that mRNA boosters provide protection against COVID-19 caused by Omicron. Previous studies of booster vaccination have demonstrated better effectiveness against hospitalization/death than against Omicron infection of any severity [26–29].
While some studies report booster effectiveness against Omicron infection compared to unvaccinated subjects [25], it is increasingly common to show VE relative to 2 doses [14, 24, 28, 30–33]. Estimates of rVE with 2 doses as reference will be somewhat lower due to better baseline protection. In the current epidemiological setting, where only a small proportion remain unvaccinated, those who have completed the primary series may be a more representative comparison group. Furthermore, subjects with a completed primary course are the target population for future booster vaccination.
In agreement with previous studies [25, 30, 33], we found substantial and rapid waning of the effectiveness against Omicron infection. In a recent surveillance report from the United Kingdom, VE estimates for mRNA booster vaccination against Omicron infection compared to unvaccinated individuals ranged from 60% to 75% in weeks 2–4 after the booster, declining to almost no effect 20 weeks after vaccination [16]. We found that the booster provided substantially better and longer-lasting protection against severe than mild disease. This gradient across self-reported severity within a generally nonhospitalized population has not been demonstrated before. Few of the severe COVID-19 cases in our study were hospitalized. This is not surprising because most participants were younger than 60 years.
Despite the clear age dependency in COVID-19 vaccination programs, the effectiveness of an mRNA booster by age is not well studied. Some studies have found a similar effect across age categories [28, 34]. In contrast, Kirsebom et al reported slightly greater waning in adults aged ≥65 years compared to 40–64 year olds [35]. Our findings also suggest a lower effectiveness in the oldest age group. However, this group was small and clear conclusions could not be drawn.
Almost 90% of the participants reported to have been tested for SARS-CoV2. The participants were much more likely to have taken an antigen test than a PCR test. The specificity of antigen tests is high, but the sensitivity is lower compared to PCR tests [36, 37]. Since January 2022, people with 3 vaccine doses are not recommended a confirmatory PCR test after a positive self-sample test [38]. As expected, booster-vaccinated participants were less likely to have had a PCR test than those with 2 doses. Consequently, the false-negative rate may be higher among booster-vaccinated participants. We cannot rule out that this may have resulted in an overestimation of the vaccine effectiveness. However, the higher rate of false negatives could have been outweighed by the higher test frequency among booster-vaccinated participants. In addition, booster-vaccinated participants were somewhat less likely to get tested due to symptoms. Thus, the detection rate for mild COVID-19 may have been higher among the booster-vaccinated participants, possibly explaining the negative booster effectiveness against mild illness observed >120 days after vaccination. We also found that women were more likely to be tested than men. Higher test frequency and selective testing may explain why the negative booster effectiveness against mild illness was limited to women.
Some studies have shown different VE by type of mRNA booster [25, 29]. In the present study, different combinations of vaccine types in the primary course were not considered, which probably limits the interpretation of the rVE according to booster type.
Nearly 95% of study participants belong to MoBa, a large, nationwide, population-based cohort that was established many years prior to the SARS-CoV-2 pandemic. In addition, we included older subjects from the newly established senior cohort. The use of electronic questionnaires made it possible to collect data on symptoms and mild infections not requiring medical care and thus not captured by patient registries. Our study covered most of the Omicron BA.1/BA.2 surge in Norway. During this period, antigen test kits were easily available and to a large extent offered for free. Another strength is the linkage to the national immunization registry, which gave detailed and complete information about vaccination dates and brand. In addition, MSIS provided reliable information about the participants’ history of positive laboratory-confirmed PCR tests for SARS-CoV-2 until February 2022. Previous SARS-CoV-2 infection influences both risk of reinfection and likelihood of receiving booster vaccination. Although we adjusted for SARS-CoV-2 infections registered in MSIS prior to start of follow-up, we have not been able to capture all infections. Among participants with no previous SARS-CoV-2 infection, the negative booster effectiveness against mild COVID-19 was attenuated but did not disappear. This suggests that some residual confounding due to previous infection remains.
Information on infections after booster vaccination, the outcome in our study, was based on self-report. Although questionnaires were sent out frequently, SARS-CoV-2 infections and COVID-19 cases are still likely to be underreported to some degree.
Booster vaccination with mRNA vaccine partly prevents moderate and severe COVID-19, but not infection with mild symptoms. Hospitalization with COVID-19 was rare in this study of subjects mainly aged 40–59 years. A better understanding of how booster vaccination can prevent moderate and severe illness without hospitalization is crucial to evaluate the full advantage of an mRNA booster. Ours is the first study to evaluate booster effectiveness against self-reported mild, moderate, and severe COVID-19 in a population-based study, giving a better assessment of the burden of disease due to Omicron infection. The results contribute valuable information to policy makers on duration of protection and thus timing of additional booster vaccinations.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are grateful to all the participants in the Norwegian Mother, Father and Child Cohort Study (MoBa) and the senior cohort. We thank the staff at the Norwegian Institute of Public Health involved in collection and preparation of data and follow-up of cohort participants.
Financial support. This work was partly supported by the Research Council of Norway through its Centers of Excellence funding scheme (project number 262700). MoBa is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research.
Supplementary Material
Contributor Information
Ida Laake, Department of Method Development and Analytics, Norwegian Institute of Public Health, Oslo, Norway.
Siri N Skodvin, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Kristine Blix, Department of Method Development and Analytics, Norwegian Institute of Public Health, Oslo, Norway.
Ida Henriette Caspersen, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Håkon K Gjessing, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway; Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway.
Lene K Juvet, Department of Infection Control and Vaccines, Norwegian Institute of Public Health, Oslo, Norway.
Per Magnus, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Siri Mjaaland, Department of Method Development and Analytics, Norwegian Institute of Public Health, Oslo, Norway.
Anna H Robertson, Department of Method Development and Analytics, Norwegian Institute of Public Health, Oslo, Norway.
Jostein Starrfelt, Department of Infection Control and Preparedness, Norwegian Institute of Public Health, Oslo, Norway.
Lill Trogstad, Department of Method Development and Analytics, Norwegian Institute of Public Health, Oslo, Norway.
Berit Feiring, Department of Method Development and Analytics, Norwegian Institute of Public Health, Oslo, Norway.
References
- 1. World Health Organization . Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern.https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern. Accessed 6 July 2022.
- 2. Brandal LT, MacDonald E, Veneti L, et al. . Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill 2021; 26:2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kared H, Wolf AS, Alirezaylavasani A, et al. . Immune responses in Omicron SARS-CoV-2 breakthrough infection in vaccinated adults. Nat Commun 2022; 13:4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Veneti L, Bøås H, Bråthen Kristoffersen A, et al. . Reduced risk of hospitalisation among reported COVID-19 cases infected with the SARS-CoV-2 Omicron BA.1 variant compared with the Delta variant, Norway, December 2021 to January 2022. Euro Surveill 2022; 27:2200077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Norwegian Institute of Public Health . Weekly reports for coronavirus and COVID-19. Week 2.https://www.fhi.no/contentassets/8a971e7b0a3c4a06bdbf381ab52e6157/vedlegg/2022/ukerapport-uke-2-10.01---16.01.22.pdf. Accessed 5 July 2022.
- 6. Norwegian Institute of Public Health . Weekly report for coronavirus and COVID-19. Weeks 23 and 24.https://www.fhi.no/contentassets/8a971e7b0a3c4a06bdbf381ab52e6157/vedlegg/2022/ukerapport-for-uke-24-13.06---19.06.22.pdf. Accessed 5 July 2022.
- 7. Feikin DR, Higdon MM, Abu-Raddad LJ, et al. . Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet 2022; 399:924–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pormohammad A, Zarei M, Ghorbani S, et al. . Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines 2021; 9:467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Andrews N, Tessier E, Stowe J, et al. . Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med 2022; 386:340–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferdinands JM, Rao S, Dixon BE, et al. . Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION network, 10 States, August 2021-January 2022. MMWR Morb Mortal Wkly Rep 2022; 71:255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Starrfelt J, Danielsen AS, Buanes EA, et al. . Age and product dependent vaccine effectiveness against SARS-CoV-2 infection and hospitalisation among adults in Norway: a national cohort study, July-November 2021. BMC Med 2022; 20:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Veneti L, Berild JD, Watle SV, et al. . Vaccine effectiveness with BNT162b2 (Comirnaty, Pfizer-BioNTech) vaccine against reported SARS-CoV-2 Delta and Omicron infection among adolescents, Norway, August 2021 to January 2022 . MedRxiv. https://doi.org/10.1101/2022.03.24.22272854v1. 25March2022, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hansen CH, Schelde AB, Moustsen-Helm IR, et al. . Vaccine effectiveness against SARS-CoV-2 infection with the Omicron or Delta variants following a two-dose or booster BNT162b2 or mRNA-1273 vaccination series: A Danish cohort study . MedRxiv. https://doi.org/10.1101/2021.12.20.21267966v3. 23December2021, preprint: not peer reviewed. [Google Scholar]
- 14. Yoon SK, Hegmann KT, Thiese MS, et al. . Protection with a third dose of mRNA vaccine against SARS-CoV-2 variants in frontline workers. N Engl J Med 2022; 386:1855–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richterman A, Behrman A, Brennan PJ, O’Donnell JA, Snider CK, Chaiyachati KH. Durability of SARS-CoV-2 mRNA booster vaccine protection against omicron among health care workers with a vaccine mandate. Clin Infect Dis 2022:ciac454. doi: 10.1093/cid/ciac454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. UK Health Security Agency . COVID-19 vaccine surveillance report. Week 24https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1083443/Vaccine-surveillance-report-week-24.pdf. Accessed 28 June 2022.
- 17. Skarbinski J, Wood MS, Chervo TC, et al. . Risk of severe clinical outcomes among persons with SARS-CoV-2 infection with differing levels of vaccination during widespread Omicron (B.1.1.529) and Delta (B.1.617.2) variant circulation in Northern California: a retrospective cohort study. Lancet Reg Health Am 2022; 12:100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kahn F, Bonander C, Moghaddassi M, et al. . Risk of severe COVID-19 from the Delta and Omicron variants in relation to vaccination status, sex, age and comorbidities—surveillance results from southern Sweden, July 2021 to January 2022. Euro Surveill 2022; 27:2200121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trogstad L, Robertson AH, Mjaaland S, Magnus P. Association between ChAdOx1 nCoV-19 vaccination and bleeding episodes: large population-based cohort study. Vaccine 2021; 39:5854–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Norwegian Institute of Public Health . Coronavirus vaccine—information for the public.https://www.fhi.no/en/id/vaccines/coronavirus-immunisation-programme/coronavirus-vaccine/. Accessed 5 July 2022.
- 21. Norwegian Directorate of Health . Brev om svar på covid-19 oppdrag nr. 620 fra Helse- og omsorgsdepartementet—Overvåkning av covid-19 del 1.https://www.helsedirektoratet.no/tema/beredskap-og-krisehandtering/koronavirus/faglig-grunnlag-til-helse-og-omsorgsdepartementet-covid-19/Oppdrag%20620%20-%20Overv%C3%A5kning%20av%20covid-19%20del%201.pdf/_/attachment/inline/86c03e90-d40a-435c-b90a-8634db8f5d18:1760fa59c87b37c65d6ff95dfd9016c49302b437/Oppdrag%20620%20-%20Overv%C3%A5kning%20av%20covid-19%20del%201.pdf. Accessed 7 July 2022.
- 22. Seppälä E, Veneti L, Starrfelt J, et al. . Vaccine effectiveness against infection with the Delta (B.1.617.2) variant, Norway, April to August 2021. Euro Surveill 2021; 26:2100793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magnus P, Birke C, Vejrup K, et al. . Cohort profile update: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2016; 45:382–8. [DOI] [PubMed] [Google Scholar]
- 24. Andrews N, Stowe J, Kirsebom F, et al. . Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med 2022; 28:831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrews N, Stowe J, Kirsebom F, et al. . Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med 2022; 386:1532–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu B, Gidding H, Stepien S, Cretikos M, Macartney K. Relative effectiveness of COVID-19 vaccination with 3 compared to 2 doses against SARS-CoV-2 B.1.1.529 (Omicron) among an Australian population with low prior rates of SARS-CoV-2 infection. Vaccine 2022; 40:6288–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meggiolaro A, Schepisi MS, Farina S, et al. . Effectiveness of vaccination against SARS-CoV-2 Omicron variant infection, symptomatic disease, and hospitalisation: a systematic review and meta-analysis. Expert Rev Vaccines 2022. doi: 10.1080/14760584.2022.2130773. [DOI] [PubMed] [Google Scholar]
- 28. Ioannou GN, Bohnert AS, O’Hare AM, et al. . Effectiveness of mRNA COVID-19 vaccine boosters against infection, hospitalization and death: a target trial emulation in the Omicron (B.1.1.529) variant era. Ann Intern Med 2022:M22-1856. doi: 10.7326/M22-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butt AA, Talisa VB, Shaikh OS, Omer SB, Mayr FB. Relative vaccine effectiveness of a SARS-CoV-2 mRNA vaccine booster dose against the Omicron variant. Clin Infect Dis 2022:ciac328. doi: 10.1093/cid/ciac328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lind ML, Robertson AJ, Silva J, et al. . Effectiveness of primary and booster COVID-19 mRNA vaccination against Omicron variant SARS-CoV-2 infection in people with a prior SARS-CoV-2 infection. MedRxiv. https://doi.org/10.1101/2022.04.19.22274056v3. 25April2022, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. . Effect of mRNA vaccine boosters against SARS-CoV-2 Omicron infection in Qatar. N Engl J Med 2022; 386:1804–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Norddahl GL, Melsted P, Gunnarsdottir K, et al. . Effect of booster vaccination against Delta and Omicron SARS-CoV-2 variants in Iceland. Nat Commun 2022; 13:5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patalon T, Saciuk Y, Peretz A, et al. . Waning effectiveness of the third dose of the BNT162b2 mRNA COVID-19 vaccine. Nat Commun 2022; 13:3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gram MA, Emborg HD, Schelde AB, et al. . Vaccine effectiveness against SARS-CoV-2 infection or COVID-19 hospitalization with the Alpha, Delta, or Omicron SARS-CoV-2 variant: a nationwide Danish cohort study. PLoS Med 2022; 19:e1003992.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirsebom F, Andrews N, Sachdeva R, Stowe J, Ramsay M, Bernal JL. Effectiveness of ChAdOx1-S COVID-19 booster vaccination against the Omicron and Delta variants in England. MedRxiv. https://doi.org/10.1101/2022.04.29.22274483v1. 01May2022, preprint: not peer reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jegerlehner S, Suter-Riniker F, Jent P, Bittel P, Nagler M. Diagnostic accuracy of a SARS-CoV-2 rapid antigen test in real-life clinical settings. Int J Infect Dis 2021; 109:118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wolfl-Duchek M, Bergmann F, Jorda A, et al. . Sensitivity and specificity of SARS-CoV-2 rapid antigen detection tests using oral, anterior nasal, and nasopharyngeal swabs: a diagnostic accuracy study. Microbiol Spectr 2022; 10:e0202921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ministry of Health and Care Services . PCR test no longer required for people with three vaccine doses.https://www.regjeringen.no/en/aktuelt/pcr-test-no-longer-required-for-people-with-three-vaccine-doses/id2898063/. Accessed 28 September 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.