Figure 7.
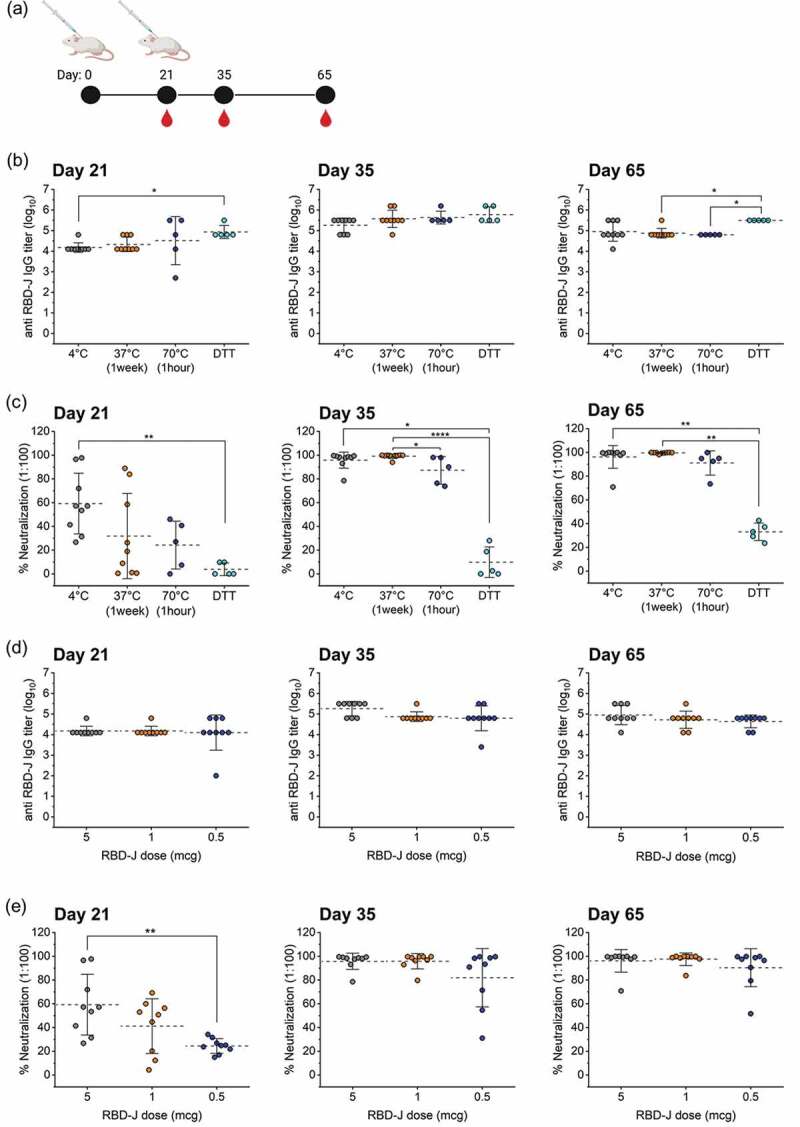
Effect of forced degradation and dose-ranging of AH+CpG–adjuvanted RBD-J on in vivo mouse immunogenicity. (a) Mice were subcutaneously immunized at day 0 and 21 and serum was collected on days 21, 35, and 65. (b) anti- RBD-J IgG titer as determined by ELISA and (c) % Neutralization at 1:100 serum dilution on day 21, 35, and 65 for mice immunized with unstressed (4°C) and stressed (37°C, 70°C and DTT-treated) AH+CpG- adjuvanted RBD-J formulations. (d) anti- RBD-J IgG titer and (e) % Neutralization at 1:100 serum dilution on day 21, 35, and 65 for mice immunized with decreasing doses of RBD-J (5, 1 and 0.5 mcg) formulated with AH+CpG adjuvants. The dashed lines represent group mean and the errors bars indicate standard deviation. P-values were determined using Kruskal–Wallis test and post hoc Dunn’s multiple comparisons test (*p ≤ 0.05; **p ≤ 0.01; ****p ≤ 0.0001). Illustration in (a) was created with Biorender.com.
