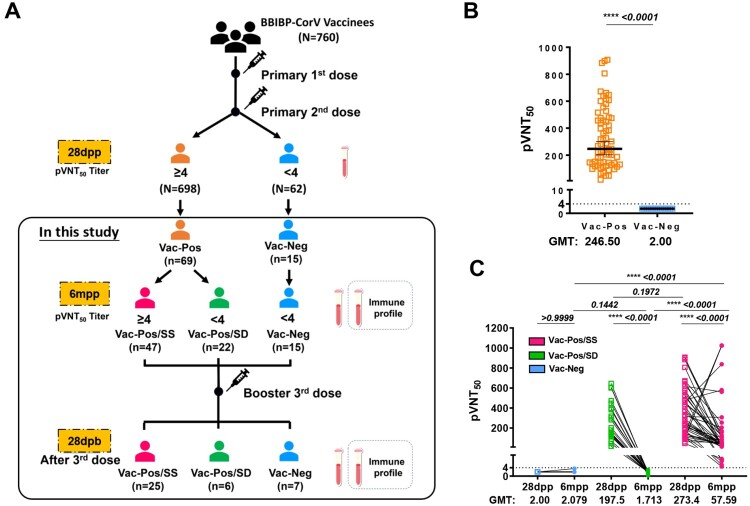
Figure 1.
Study design and two-dose BBIBP-CorV vaccination elicit diverse humoral immune responses. (A) Diagram of the study groups. A total of 84 participants were included for the analysis of virus-specific neutralizing antibodies 28 days (28dpp) and 6 months (6mpp) after the completion of the vaccination regimen. Immune profiles were also measured on 6mpp. Forty-seven participants exhibited a sustained level of neutralizing antibodies (Vac-Pos/SS); 22 participants lost their neutralizing antibody level at 6mpp (Vac-Pos/SD); 15 participants never showed the presence of neutralizing antibody (Vac-Neg). (B) Levels of wildtype (WT) spike (S)-specific neutralization antibody were compared between Vac-Pos and Vac-Neg groups at 28dpp. The lowest serum dilution tested was 1:4; undetectable pVNT50 values were set at a pVNT50 of 2. GMTs are indicated above the graph. (C) Levels of WT S-specific neutralizing antibody were analysed early (28dpp) and late (6mpp) after the 2-dose vaccination. Lines and bars indicated median with interquartile range (IQR). P value determined by unpaired t-test (B) or by paired t-test (C).