Abstract
The objectives of this study were to examine levels of arsenic, cadmium, lead, mercury and selenium in edible tissue of seven species of marine fish collected from several Aleutian islands (in 2004) to determine: 1) interspecific differences, 2) locational differences (among Aleutian Islands), 3) size-related differences in any metal levels within a species, and 4) potential risk to the fish or to predators on the fish, including humans. We also compared metals levels to those of three other fish species previously examined in detail, as well as examining metals in the edible tissue of octopus (Octopus dofleini). Octopus did not have the highest levels of any metal. There were significant interspecific differences in all metal levels among the fish species, although the differences were less than an order of magnitude, except for arsenic (mean of 19,500 ppb in Flathead sole, Hippoglossoides elassodon). Significant intraisland variation occurred among the four sites on Amchitka, but there was not a consistent pattern. There were significant interisland differences for some metals and species. Mercury levels increased significantly with size for several species; lead increased significantly for only one fish species; and cadmium and selenium decreased significantly with size for halibut (Hippoglossus stenolepis). The Alaskan Department of Health and Social Services supports unrestricted consumption of most Alaskan fish species for all people, including pregnant women. Most mean metal concentrations were well below the levels known to adversely affect the fish themselves, or predators that consume them (including humans), except for mercury in three fish species (mean levels just below 0.3 ppm), and arsenic in two fish species. However, even at low mercury levels, people who consume fish almost daily will exceed guideline values from the Centers for Disease Control and the Environmental Protection Agency.
Keywords: Human health risk, mercury, cadmium, lead, fish
1. Introduction
Coastal environments are subject to metal contamination from natural sources, as well as from various point and non-point source pollution (Buck et al., 2005; Li et al., 2012). Movement of metals and other contaminants in coastal systems has been extensively studied, especially for mercury (Fitzgerald, 1989; Fitzgerald et al., 2005). Open ocean waters are expected to have lower levels of anthropogenic contaminants because they are distant from point-source pollution, run-off from land, and river-borne contaminants. The waters around oceanic islands far from mainland areas present a unique situation in that there is some run-off and potential point-source pollution from human activities on the islands, but they are generally surrounded by uncontaminated marine waters. In general, metal levels in sediment (and thus in the food chain) are generally lower in pristine environments than in urban areas (Meador et al. 1998).
Metal bioaccumulation in fish can be used as bioindicators of metal exposure that can directly affect the fish themselves, or can be transferred up the food web to larger organisms, including humans (Burger 2006). Fishing has important cultural and economic aspects, as well as providing protein for coastal and riverine peoples (Toth and Brown, 1997). High fishing rates occur in both rural and urban areas (Burger et al., 2001a, b; Bienenfeld et al., 2003), among Native Americans (Harris and Harper, 2000; Burger et al., 2007a,b), and in many regions of the world (Burger et al., 2003; Takezaki et al., 2003; Lu et al., 2008; Hsiao et al., 2011). Since well over half of the US and the world’s population lives near the coasts (NOAA, 2012), saltwater fish are an important food source. Fish also provide fish oil for humans, fishmeal for aquaculture use (Brunner et al., 2009), recreational opportunities, cultural benefits, and aesthetic pleasures (Egeland and Middaugh, 1997; Toth and Brown, 1997; Harris and Harper, 1998; Burger, 2000, 2002).
For many parts of the world fish and shellfish are a critical and important source of protein (Dorea et al., 1998; Pinheiro et al., 2009), and many people are faced with deciding whether the benefits of eating fish outweigh the risks from contaminants (Gochfeld and Burger, 2005).. Balancing benefits and risks has been particularly important for subsistence cultures, and Alaska has taken a position that the benefits generally outweigh the risks (Egeland and Middaugh 1997), and has historically recommended unrestricted fish consumption for most species of Alaska fish (Verbrugge 2007). Fish consumption is associated with low blood cholesterol (Anderson and Wiener, 1995), positive pregnancy outcomes, and better child cognitive test performances (Oken et al., 2008). Fish and fish oil contain omega-3 (n-3) fatty acids that reduce cholesterol levels and the incidence of heart disease, blood pressure, stroke, and pre-term delivery (Patterson, 2002; Virtanen et al., 2008; Ramel et al., 2010).
Some fish contain contaminants (methylmercury [MeHg], PCBs, other metals) at high enough levels to cause effects on the fish themselves (Eisler, 1987), and on top-level predators, including humans (WHO, 1989; NRC, 2000; Hightower and Moore, 2003). Fish consumption is the most significant source of methylmercury exposure for other vertebrates (Eisler, 1987) and for the public (Grandjean et al., 1997; Rice et al., 2000). Methylmercury in some fish can cause adverse health effects in people consuming large quantities (IOM, 1991, 2006; Grandjean et al., 1997; Gochfeld, 2003; Hites et al., 2004). Effects include neurodevelopmental deficits in fetuses, and behavioral deficits and poorer cognitive test performance in young children (NRC, 2000; JECFA, 2003; Oken et al., 2008; Freire et al., 2010). In adults, mercury exposure counteracts the cardioprotective effects of fish consumption, promotes cardiovascular disease, and results in neurological deficits (Guallar et al., 2002; Hightower and Moore, 2003; Choi et al., 2009).
The risk to consumers from contaminants in fish can be reduced by source reduction, altering the rate of methylation, and by changing patterns of species preferentially consumed. Countries respond to high mercury levels in fish by issuing consumption advisories or fishing bans. In the United States, it is largely the responsibility of states to determine health risks and to issue fish and shellfish consumption advisories. The process of controlling fish consumption, and providing fish consumption advisories, is more difficult for remote islands with low density human populations, as occurs in the Aleutian Islands. It is further complicated by the reliance of island dwellers on local resources. Such subsistence fishers and hunters must rely on local resources and their fishing activity depends upon weather constraints, in a harsh sub-arctic ecosystem (Nygard et al., 2001; Patrick, 2002; Hamrick and Smith, 2003, Burger et al., 2007c). In addition, about half of the fish and shellfish sold in the continental United States comes from the Bering Sea (AFSC, 2003). Cod, which mainly comes from the Bering Sea and North Pacific, is on the top 10 U.S. per capita fish consumption list, and has been for the last ten years (Fishwatch, 2013, NOAA, 2010). One-third of the salmon eaten in the U.S. comes from Alaska (Fishwatch, 2013). There are also important trawl fisheries for rockfish, rock sole, and flathead sole in Alaska, although rockfish is often bycatch from the Atka mackerel (Pleurogrammus monopteryglius) fishery (Clausen and Heifetz, 2002). Dutch Harbor- Unalaska (Aleutians) often is the leading “landed fish” port in the World (NOAA, 2013). The U.S. is the third largest seafood market in the World, after Japan and China (CAAF, 2013). Thus the Bering Sea ecosystem is important economically for food chain effects and for people who eat marine fish.
This study examined metal levels in several fish species and Pacific Octopus from the Aleutian Islands in the Bering Sea (NRC, 1996, scientific names given in Table 1, Fig. 1).
Table 1.
Contamininant levels in 10 species collected from from the Aleutian Islands during the summer of 2004. Given are means±SE and geometric means below. Comparisons are made with Kruskal-Wallis 1-Way ANOVA, yielding an X2 statistic. All values are in ng/g (ppb wet weight). Trophic level, maximum life span, maturity age, and prey items are from www.fishbase.org.
| N | Trophic Level | Max Life Span | Age at Maturity | Prey Items | Total Length (cm) | Standard Length (cm) | Weight (g) | Arsenic | Cadmium | Lead | Mercury | Selenium | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Black Rockfish (Sebastes melanops) |
34 | 4.4 | 50+ | 9–13 | Fish, zooplankton, zoobenthos | 38.3 ± 0.77 | 33.6 ± 0.78 | 914 ± 60.5 | 276 ± 38.6 | 17.6 ± 11.2 | 1.60 ± 0.8 | 145 ± 17.9 | 527 ± 21.9 |
| 38.1 | 33.3 | 861 | 201 | 5.02 | 0.14 | 113 | 509 | ||||||
| Dolly Varden (Salvelinus malma) |
75 | 4.2 | 12 | 3–5 | Zoobenthos, zooplankton, fish | 32.4 ± 0.81 | 28.9 ± 0.71 | 330 ± 22.5 | 69.3 ± 12.3 | 2.10 ± 1.10 | 22.1 ± 4.80 | 114 ± 12.9 | 349 ± 35.7 |
| 31.6 | 28.2 | 269 | 5.70 | 0.07 | 0.96 | 48.2 | 133 | ||||||
| Pacific Halibut (Hippoglossus stenolepis) |
24 | 4.1 | 55 | 5–20 | Zoobenthos, zooplankton, fish | 76.0 ± 7.20 | 67.4 ± 6.50 | 8640 ± 2160 | 636 ± 120 | 10.4 ± 3.80 | 28.4 ± 9.79 | 158 ± 43.5 | 373 ± 29.3 |
| 68.6 | 60.2 | 3600 | 408 | 2.2 | 4.13 | 93.7 | 348 | ||||||
| Great Sculpin (Myoxocephalus polyacanthocephalus) |
17 | 4.1 | 9–13 | 6–8 | Zoobenthos, fish | 44.9 ± 1.96 | 40.5 ± 1.14 | 1720 ± 118 | 1400 ± 221 | 4.23 ± 1.41 | 11.8 ± 3.50 | 294 ± 53.7 | 613 ± 35.2 |
| 43.9 | 40.2 | 1657 | 1173 | 1.60 | 1.90 | 228 | 596 | ||||||
| Pacific Cod (Gadus macrocephalus) |
140 | 4.0 | 18–25 | 2–8 | Zoobenthos, fish | 61.7 ± 1.65 | 56.5 ± 1.50 | 4090 ± 436 | 2180 ± 231 | 8.6 ± 0.96 | 60.9 ± 5.9 | 173 ± 11.5 | 183 ± 6.6 |
| 59.0 | 54.1 | 2340 | 1590 | 1.90 | 23.3 | 128 | 165 | ||||||
| Rock Greenling (Hexagrammos lagocephalus) |
43 | 3.9 | 11 | 3.5 | Zoobenthos, zooplankton, fish | 35.4 ± 0.61 | 30.6 ± 0.59 | 611 ± 31.2 | 381 ± 51.4 | 1.98 ± 0.30 | 16 ± 3.78 | 99.0 ± 14.1 | 188 ± 16.1 |
| 35.2 | 30.3 | 575 | 239 | 0.43 | 2.19 | 49.6 | 85.9 | ||||||
| Yellow Irish Lord (Hemilepidotus jordani) |
43 | 3.8 | 12–13 | 4–7 | Zoobenthos, fish | 41.5 ± 0.63 | 34.0 ± 0.54 | 890 ± 42.9 | 564 ± 48.9 | 6.54 ± 0.96 | 52 ± 12.6 | 272 ± 28.7 | 321 ± 18.9 |
| 41.2 | 33.8 | 850 | 420 | 3.58 | 23.1 | 198 | 298 | ||||||
| Pink Salmon (Oncorhynchus gorbuscha) |
15 | 4.2 | 3 | 2 | Zoobenthos, zooplankton, fish | 212 ± 45.5 | 2.74 ± 0.94 | 27 ± 11.5 | 41.9 ± 5.3 | 246 ± 26.4 | |||
| 159 | 0.41 | 12.4 | 33.7 | 218 | |||||||||
| Flathead Sole (Hippoglossoides elassodon) |
39 | 3.6 | 27 | 1–3 | Zoobenthos, fish | 38.8 ± 0.5 | 32.9 ± 0.4 | 588 ± 24.4 | 19500 ± 1010 | 4.2 ± 0.5 | 50.3 ± 8.0 | 276 ± 12.7 | 398 ± 37.4 |
| 38.7 | 32.7 | 568 | 18500 | 2.40 | 40.5 | 265 | 338 | ||||||
| Rock Sole (Lepidopsetta bilineate) |
8 | 3.2 | 26 | 9 | Zoobenthos, zooplankton, fish | 35.6 ± 2.2 | 30.0 ± 1.5 | 574 ± 107 | 4340 ± 693 | 8.76 ± 3.64 | 43 ± 16.3 | 95.4 ± 22.7 | 374 ± 53.5 |
| 35.2 | 29.7 | 509 | 3950 | 0.04 | 28.5 | 83.1 | 348 | ||||||
|
| |||||||||||||
| X 2 | 282 (<0.0001) | 94.7 (0.0005) | 142 (<0.0001) | 117 (<0.0001) | 168 (<0.0001) | ||||||||
Figure 1.
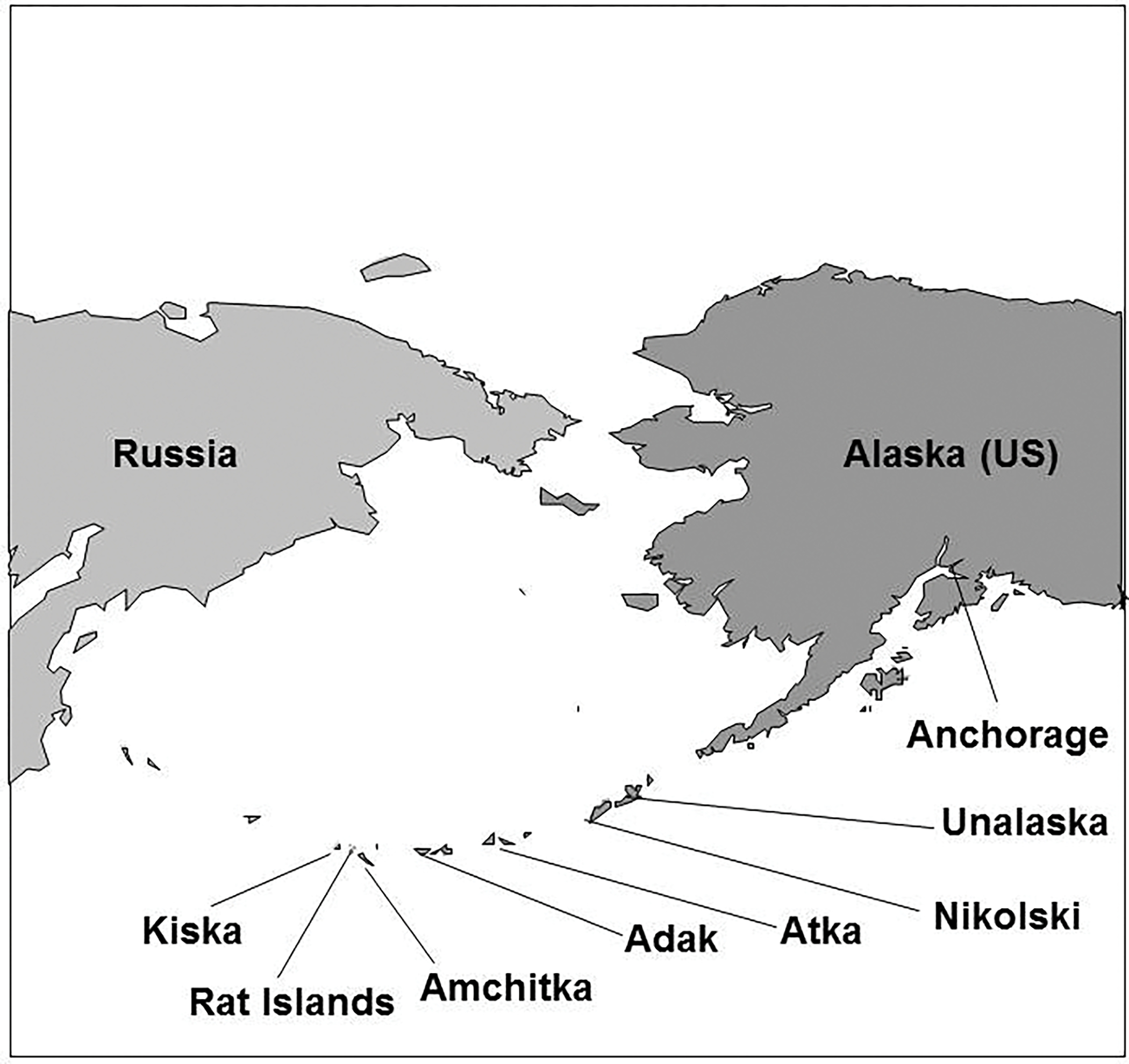
Map of the Aleutian Chain of Alaska, showing the islands where samples were collected.
Specimens were collected on the Amchitka Expedition (2004–2005) operated by the Consortium for Risk Evaluation with Stakeholder Participation (CRESP) (Powers et al., 2005), under appropriate permits from the State of Alaska’s Department of Fish and Game (# CF-04-043; Fig. 1), and with an appropriate Rutgers University Animal Review Board Protocol (#17-097). The objectives of this paper are to examine levels of arsenic, cadmium, lead, mercury and selenium in edible tissue of seven species of fish (and 3 others previously published) from the waters of several islands in the Aleutians of Alaska, and to determine: 1) interspecific differences, 2) locational differences, and 3) size relationships for metals. Levels in edible tissue of Pacific octopus (Octopus dofleini) are also reported. More detailed analysis of Pacific cod, great sculpin, and flathead sole can be found in Burger and Gochfeld (2007) and Burger et al. (2007a–c), but are presented here in Table 1 for completeness.
The chain of Aleutian Islands juts out from Alaska toward Siberia, and some of the islands are closer to Russia than to Alaska (Fig. 1). Some islands are quite small and isolated in one of the most dangerous and rugged seas (Bering Sea). Some islands are inhabited by Alaskan Natives (Aleuts = Unangan) who rely on subsistence foods throughout the year to supplement limited commercial foods brought by small plane (up to twice weekly) or ship (3–4 times a year). Nikolski (Umnak Island) is reportedly the oldest continually-occupied community in North America (Black, 1974; Schlung, 2003). Aleut fishermen from Atka sometimes go as far as Amchitka to catch some fish, particularly halibut.
From 1965–1971 the United States conducted three underground nuclear tests on Amchitka (Long Shot 1965=80 kilotons, Milrow 1969=1 megaton, Cannikin 1971= 5 megatons). In 2003–2005, at the request of the Governor of Alaska and various agencies, the multi-university, interdisciplinary CRESP conducted research related to marine contamination around Amchitka, including a Geophysical and Biological Expedition in 2004. Various marine species were analyzed for radionuclides (CRESP 2005 for results). Adak and Kiska served as reference sites for the Amchitka samples. Since metals pose a risk to humans, this paper provides this data to complement the radionuclide data.
2. Materials and methods
Specimens were collected under appropriate permits from the State of Alaska’s Department of Fish and Game (# CF-04-043; Fig. 1). Fish and octopus were collected in July and August 2004 from the Aleutian islands of Adak (52° N lat; 176° W long), Amchitka (51° N lat; 179° E long), Rat Island (51.9°N lat: 178.2°E long), and Kiska (51° N lat;177° E long), and fish were collected in spring 2005 from Nikolski on Umnak Island (53 N lat; 169° W long). Pink salmon samples were also collected from Atka (52 N lat; 174 W long) in August 2003. Fish samples were collected from Bering Sea locations closest to the Long Shot and Cannikin test sites and from North Pacific waters closest to Milrow (Figure 1) where surface or subsurface contamination from the bomb tests might be anticipated to occur. Amchitka and Kiska Islands are part of the Alaska Maritime National Wildlife Refuge that was established in 1913 (ATSDR, 2004). There are small Aleut communities on Adak (ca 300 people), Umnak (ca40) and Atka (ca 85), but Amchitka and Kiska are currently uninhabited although they are traditional Aleut homelands.
Fish were collected from all five islands either from land or boat, with rod and reel by Aleuts and by scientists, and by trawling, and by underwater spearing, while based on the trawlers, Ocean Explorer or Gladiator. Aleuts from Nikolski (Umnak Island) and Adak were on the expedition to Amchitka and Kiska, and collected samples in the traditional manner in all locations. Fish were immediately measured, weighed and dissected, and samples of muscle were frozen for later analysis. All samples were given a unique number and had Chain of Custody forms with the following information recorded: specimen number, species, date, island, location from that island, collector, and preparator. All samples were shipped frozen to the Environmental and Occupational Health Sciences Institute (EOHSI) of Rutgers University for metal analysis. Most samples were collected as part of research by the Consortium for Risk Evaluation with Stakeholder Participation (CRESP) to examine radionuclide levels in marine biota for the Department of Energy (Burger et al., 2007a). Levels of all anthropogenic radionuclides examined were well below safe human health risk guidance levels (Powers et al., 2005). Scientific names for all fish species are given in Table I. All samples were analyzed in the Elemental Analysis Laboratory of the Environmental and Occupational Health Sciences Institute of Rutgers University in Piscataway, NJ. Total mercury was analyzed by cold vapor technique, and the other elements were analyzed by graphite furnace atomic absorption (Burger and Gochfeld, 1991). Metal speciation was not conducted. All concentrations are expressed in ng/g (ppb) on a wet weight basis. All samples were analyzed in batches with calibration standards, blanks, spiked specimens, and DORM-2 Certified Reference Material. Instrument detection limits were 0.2 ppb for arsenic, 0.02 ppb for cadmium, 0.08 ppb for chromium, 0.15 ppb for lead, 0.7 for selenium, and 0.2 ppb for mercury. The accepted recoveries for reference material and spikes ranged from 85% to 115%; no batches were outside of these limits.
Non-parametric Kruskal-Wallis one way analysis of variance (SAS PROC NPAR1WAY with Wilcoxon option yielding a chi square statistic) was used to examine differences among species and islands. Both arithmetic and geometric means are given in tables to facilitate comparisons with other studies. We accept a probability level of 0.05 as significance, but present all values below 0.10 to allow the reader to assess the significance themselves. Because three species were examined in detail elsewhere (Pacific cod, great sculpin, flathead sole; Burger and Gochfeld, 2007; Burger et al., 2007a–c), the relationship between size and metals for those species is not discussed in this paper.
3. Results
3.1. Interspecific differences
There were body size, prey, and trophic level differences in the fish examined (Table 1). Halibut was the largest, followed by Pacific cod (Burger et al. 2007c). The others were smaller and similar in size. The species differ greatly in typical life span. There were significant interspecific differences in all metals for fish (Table 1), although means were within an order of magnitude for mercury, selenium, and cadmium. The levels that follow are all nanograms per gram (ppb) wet weight. Mean arsenic levels ranged from 69.3 ppb (Dolly Varden) to 19,500 ppb (flathead sole). Flathead sole and rock sole (4340 ppb) had much higher arsenic levels than other species. The mean levels for cadmium ranged from 2.1 ppb (Dolly Varden) to 17.6 ppb (black rockfish). Mean lead varied from 1.6 ppb (black rockfish) to 60.9 ppb (Pacific cod). Mean levels for mercury ranged from 49.9 ppb (pink salmon) to to 294 for great sculpin. Mean levels for selenium ranged from 183 ppb for Pacific cod to 613 ppb for great sculpin. (Table 1).
Because octopus is a delicacy, we examined metal levels in the 5 specimens collected from Amchitka. Mean values (±SE) were as follows: arsenic = 2,160 ± 225 ppb, cadmium = 47.0 ± 29.1 ppb, lead = 20.6 ± 11.3 ppb, mercury = 38.2 ± 10.5 ppb, and selenium = 231± 35.1 ppb. There was a great deal of size differences between the octopus collected (mean weight = 18,600 ± 8,615 gr).
3.2. Intraisland differences
Amchitka was the primary target of the expedition, and a systematic effort was made to collect fish from the coastal waters closest to the nuclear test sites where impact of any former or current “leakage” of contaminants might be detected. Radionuclide results have been reported separately (Powers et al. 2005). It was not possible to obtain all species at all locations. Black rockfish were collected at four locations on Amchitka (off Constantine Harbor, Cannikin, and Long Shot on the Bering Sea side, and off Milrow on the Pacific side) and at Kiska (Table 2). Among sites black rockfish (n=24) did not vary in size or lead levels and mercury was marginally (p=0.07) higher at Long Shot. The other elements varied significantly but inconsistently (Table 2) with arsenic highest at Cannikin and cadmium and selenium, highest at the Harbor. Rock greenling (n=25) did not vary in size among sites, and only lead varied with more than an order of magnitude difference between Cannikin (33 ppb) and the Harbor (1 ppb; p=0.02, Table 3). Yellow Irish lord did not vary in size among sites, but lead varied by an order of magnitude between Cannikin (158 ppb) and Long Shot (15 ppb; p=0.04). Selenium also varied among sites (p=0.03, Table 4). Halibut (n=7) were marginally larger from Long Shot (mean 134cm) compared with Milrow (81 ppm, p=0.05), and the former fish had higher mercury (662 ppb vs 189 ppb), but the sample sizes were too small to detect significant differences among sites for any elements (Table 5). Dolly Varden were collected in fresh water, and the Cannikin Lake fish (n=21) were significantly larger than those from Fox Lake (n=30) adjacent to the Harbor. Surprisingly average mercury levels were identical, while arsenic, cadmium, and selenium were all significantly higher in the Cannikin Lake sample (Table 6).
Table 2.
Contaminant levels in Black Rockfish collected from from the Aleutian Islands during the summer of 2004. Given are means±SE and geometric means below. Comparisons are made with Kruskal-Wallis 1-Way ANOVA, yielding an X2 statistic. All values are in ng/g (ppb wet weight).
| Black Rockfish | Total Length (cm) | Standard Length (cm) | Weight (g) | Arsenic | Cadmium | Lead | Mercury | Selenium | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | |||||||||
| Amchitka Island | |||||||||
| Constantine Harbor | 4 | 42.8 ± 0.85 | 36.5 ± 1.04 | 1160 ± 42.4 | 218 ± 30.9 | 5.75 ± 1.11 | 0.08 ± 0.00 | 122 ± 44.6 | 658 ± 24.8 |
| 42.7 | 36.5 | 1160 | 211 | 5.40 | 0.08 | 93.0 | 656 | ||
| Cannikin | 7 | 34.6 ± 1.75 | 33.0 ± 2.90 | 741 ± 83.3 | 533 ± 125 | 2.00 ± 0.44 | 2.63 ± 1.77 | 113 ± 29.4 | 434 ± 44.6 |
| 34.3 | 32.3 | 710 | 457 | 1.74 | 0.29 | 89.0 | 420 | ||
| Long Shot | 3 | 40.7 ± 1.96 | 34.8 ± 1.64 | 1130 ± 156 | 269 ± 141 | 133 ± 126 | 0.08 ± 0.00 | 365 ± 61.8 | 552 ± 62.7 |
| 40.6 | 34.8 | 1110 | 207 | 24.0 | 0.08 | 353 | 545 | ||
| Milrow | 10 | 39.0 ± 1.50 | 33.3 ± 1.3 | 948 ± 163 | 147 ± 31.2 | 3.90 ± 0.94 | 3.46 ± 2.49 | 123 ± 22.8 | 490 ± 43.5 |
| 38.7 | 33.1 | 876 | 102 | 3.50 | 0.22 | 104 | 468 | ||
|
| |||||||||
| Intra-island Kruskal-Wallis | 9.8 (0.02) | 5.3 (NS) | 10.7 (0.01) | 11.6 (0.009) | 8.3 (0.04) | 2.0 (NS) | 7.1 (0.07) | 9.6 (0.02) | |
| Amchitka (Totals) | 24 | 38.5 ± 1.00 | 33.9 ± 1.02 | 946 ± 79.8 | 287 ± 52.3 | 19.8 ± 15.8 | 2.23 ± 1.16 | 150 ± 22.9 | 509 ± 27.6 |
| 38.2 | 33.6 | 889 | 195 | 3.93 | 0.17 | 114 | 489 | ||
|
| |||||||||
| Kiska Island | 10 | 37.9 ± 1.11 | 32.7 ± 1.05 | 838 ± 81.9 | 250 ± 41.2 | 12.4 ± 3.74 | 0.08 ± 0.00 | 133 ± 28.0 | 570 ± 31.8 |
| 37.7 | 32.5 | 798 | 216 | 8.80 | 0.08 | 112 | 562 | ||
|
| |||||||||
| X 2 | 10.6 (0.03) | 5.8 (NS) | 10.7 (0.03) | 12.5 (0.01) | 14.6 (0.006) | 4.6 (NS) | 7.4 (NS) | 10.7 (0.03) | |
Table 3.
Contaminant levels in Rock Greenling collected from from the Aleutian Islands during the summer of 2004. Given are means±SE and geometric means below. Comparisons are made with Kruskal-Wallis 1-Way ANOVA, yielding an X2 statistic. All values are in ng/g (ppb wet weight).
| Rock Greenling | Total Length (cm) | Standard Length (cm) | Weight (g) | Arsenic | Cadmium | Lead | Mercury | Selenium | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | |||||||||
| Amchitka Island | |||||||||
| Cannikin | 8 | 35.8 ± 1.37 | 31.4 ± 1.15 | 619 ± 51.5 | 273 ± 94.2 | 2.22 ± 0.74 | 33.0 ± 10.3 | 117 ± 29.4 | 208 ± 47.3 |
| 35.6 | 31.2 | 604 | 119 | 0.95 | 12.7 | 52.0 | 59.8 | ||
| Constantine Harbor | 5 | 32.5 ± 1.32 | 28.6 ± 1.48 | 504 ± 42.6 | 445 ± 88.0 | 2.13 ± 1.02 | 0.95 ± 0.70 | 97.4 ± 22.4 | 252 ± 33.6 |
| 32.4 | 28.4 | 497 | 406 | 0.32 | 0.30 | 87.0 | 244 | ||
| Long Shot | 2 | 31.0 ± 2.00 | 27.0 ± 1.00 | 459 ± 65.5 | 437 ± 238 | 2.23 ± 0.75 | 6.84 ± 1.33 | 76.5 ± 41.5 | 231 ± 0.67 |
| 30.9 | 27.0 | 554 | 366 | 2.10 | 6.70 | 64.3 | 231 | ||
| Milrow | 10 | 35.5 ± 1.09 | 30.8 ± 1.11 | 603 ± 56.0 | 269 ± 54.5 | 2.42 ± 0.48 | 5.17 ± 1.81 | 153 ± 45.6 | 159 ± 33.0 |
| 35.4 | 30.6 | 579 | 209 | 1.17 | 1.53 | 94.3 | 110 | ||
|
| |||||||||
| Intra-island Kruskal-Wallis | 4.9 (NS) | 3.6 (NS) | 2.8 (NS) | 3.0 (NS) | 0.4 (NS) | 10.3 (0.02) | 0.7 (NS) | 2.0 (NS) | |
| Amchitka (Totals) | 25 | 34.6 ± 0.73 | 30.2 ± 0.68 | 577 ± 30.3 | 319 ± 44.4 | 2.28 ± 0.35 | 13.4 ± 4.26 | 124 ± 21.0 | 199 ± 21.5 |
| 34.4 | 30.0 | 558 | 208 | 0.89 | 2.39 | 74.4 | 113 | ||
|
| |||||||||
| Adak Island | 4 | 38.1 ± 0.8 | 33.9 ± 0.75 | 815 ± 68.1 | 436 ± 88.5 | 1.51 ± 0.86 | 30.3 ± 15.8 | 120 ± 25.5 | 173 ± 21.5 |
| 38.1 | 33.9 | 807 | 409 | 0.17 | 6.40 | 114 | 169 | ||
| Umnak Island | 4 | 41.8 ± 1.31 | 36.3 ± 1.11 | 943 ± 120 | 910 ± 226 | 0.22 ± 0.21 | 44.5 ± 22.1 | 52.5 ± 9.12 | 236 ± 63.5 |
| 41.7 | 36.2 | 917 | 817 | 0.03 | 10.0 | 49.4 | 214 | ||
| Kiska Island | 10 | 33.8 ± 1.03 | 27.9 ± 0.97 | 482 ± 43.5 | 301 ± 133 | 2.14 ± 0.84 | 3.81 ± 1.93 | 46.0 ± 17.7 | 147 ± 34.2 |
| 33.6 | 27.7 | 450 | 165 | 0.30 | 0.62 | 13.0 | 23.2 | ||
|
| |||||||||
| X 2 | 17.0 (0.009) | 18.4 (0.005) | 17.0 (0.009) | 12.7 (0.05) | 5.7 (NS) | 14.0 (0.03) | 10.4 (NS) | 4.6 (NS) | |
Table 4.
Contaminant levels in Yellow Irish Lord collected from from the Aleutian Islands during the summer of 2004. Given are means±SE and geometric means below. Comparisons are made with Kruskal-Wallis 1-Way ANOVA, yielding an X2 statistic. All values are in ng/g (ppb wet weight).
| Yellow Irish Lord | Total Length (cm) | Standard Length (cm) | Weight (g) | Arsenic | Cadmium | Lead | Mercury | Selenium | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | |||||||||
| Amchitka Island | |||||||||
| Cannikin | 5 | 40.8 ± 2.20 | 33.5 ± 1.91 | 751 ± 129 | 485 ± 70.7 | 9.00 ± 4.28 | 158 ± 74.5 | 183 ± 56.0 | 293 ± 48.4 |
| 40.6 | 33.3 | 708 | 467 | 6.51 | 84.4 | 148 | 275 | ||
| Constantine Harbor | 4 | 43.1 ± 1.78 | 35.8 ± 1.55 | 1190 ± 153 | 445 ± 114 | 4.25 ± 0.95 | 24.5 ± 5.58 | 245 ± 80.6 | 299 ± 24.3 |
| 43.0 | 35.7 | 1160 | 399 | 3.98 | 22.1 | 207 | 296 | ||
| Long Shot | 5 | 45.0 ± 1.96 | 36.6 ± 1.65 | 1090 ± 104 | 511 ± 126 | 4.60 ± 1.63 | 14.7 ± 3.71 | 287 ± 77.7 | 424 ± 15.5 |
| 44.8 | 36.4 | 1070 | 463 | 5.00 | 12.8 | 253 | 423 | ||
| Milrow | 3 | 37.7 ± 0.88 | 31.2 ± 0.60 | 676 ± 42.3 | 441 ± 149 | 13.3 ± 1.86 | 65.9 ± 24.0 | 89.7 ± 26.7 | 243 ± 26.3 |
| 37.6 | 31.2 | 674 | 396 | 13.1 | 58.5 | 82.7 | 239 | ||
|
| |||||||||
| Intra-island Kruskal-Wallis | 5.3 (NS) | 4.6 (NS) | 7.8 (0.05) | 0.6 (NS) | 6.1 (NS) | 8.4 (0.04) | 6.0 (NS) | 9.0 (0.03) | |
| Amchitka (Totals) | 17 | 42.0 ± 1.10 | 34.5 ± 0.91 | 941 ± 76.5 | 476 ± 51.5 | 7.35 ± 1.54 | 68.2 ± 25.5 | 212 ± 35.7 | 324 ± 22.7 |
| 41.8 | 34.3 | 889 | 436 | 6.15 | 33.1 | 169 | 310 | ||
|
| |||||||||
| Adak Island | 2 | 34.8 ± 4.25 | 29.0 ± 3.50 | 679 ± 182 | 271 ± 271 | 3.51 ± 3.50 | 2.37 ± 2.29 | 42.0 ± 38.0 | 143 ± 70.6 |
| 34.5 | 28.8 | 654 | 7.36 | 0.27 | 0.59 | 17.9 | 125 | ||
| Umnak Island | 9 | 41.8 ± 0.53 | 33.7 ± 0.47 | 808 ± 55.8 | 925 ± 140 | 5.11 ± 2.22 | 84.8 ± 31.2 | 248 ± 35.6 | 341 ± 48.9 |
| 41.8 | 33.6 | 795 | 320 | 1.68 | 50.8 | 224 | 320 | ||
| Kiska Island | 15 | 41.5 ± 1.06 | 34.2 ± 1.00 | 910 ± 76.7 | 487 ± 47.7 | 6.87 ± 1.65 | 19.6 ± 3.41 | 385 ± 56.7 | 330 ± 35.0 |
| 41.3 | 34.0 | 872 | 454 | 4.47 | 15.6 | 303 | 307 | ||
|
| |||||||||
| X 2 | 9.7 (NS) | 9.4 (NS) | 12.6 (0.05) | 11.2 (0.08) | 7.0 (NS) | 20.1 (0.003) | 14.4 (0.03) | 12.2 (0.06) | |
Table 5.
Contaminant levels in Halibut collected from from the Aleutian Islands during the summer of 2004. Given are means±SE and geometric means below. Comparisons are made with Kruskal-Wallis 1-Way ANOVA, yielding an X2 statistic. All values are in ng/g (ppb wet weight).
| Halibut | Total Length (cm) | Standard Length (cm) | Weight (g) | Arsenic | Cadmium | Lead | Mercury | Selenium | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | |||||||||
| Amchitka Island | |||||||||
| Long Shot | 2 | 149 ± 13.0 | 134 ± 11.5 | 26310 ± 19100 | 513 ± 309 | 0.19 ± 0.18 | 10.5 ± 1.63 | 662 ± 267 | 302 ± 71.3 |
| 148 | 133 | 18100 | 409 | 0.06 | 10.4 | 605 | 294 | ||
| Milrow | 5 | 91.4 ± 9.9 | 81.0 ± 7.5 | 9780 ± 3107 | 316 ± 97.4 | 6.80 ± 3.40 | 34.9 ± 15.9 | 189 ± 121 | 274 ± 36.7 |
| 91.6 | 73.6 | 6640 | 235 | 2.40 | 10.3 | 39.0 | 284 | ||
|
| |||||||||
| Intra-island Kruskal-Wallis | 3.8 (0.05) | 3.8 (0.05) | 1.3 (NS) | 0.6 (NS) | 2.4 (NS) | 0.2 (NS) | 2.4 (NS) | 0.6 (NS) | |
| Amchitka (Totals) | 7 | 108 ± 12.9 | 96.0 ± 11.3 | 14500 ± 5580 | 372 ± 102 | 4.91 ± 2.65 | 27.9 ± 11.8 | 324 ± 134 | 282 ± 30.2 |
| 103 | 92.3 | 10500 | 285 | 1.13 | 8.9 | 128 | 272 | ||
|
| |||||||||
| Rat Island | 6 | 51.0 ± 11.1 | 43.8 ± 9.90 | 3010 ± 2400 | 566 ± 133 | 32.4 ± 11.0 | 1.29 ± 1.22 | 78.3 ± 1.38 | 552 ± 56.5 |
| 46.9 | 40.1 | 1020 | 418 | 20.1 | 0.20 | 78.3 | 537 | ||
| Kiska Island | 11 | 69.5 ± 7.7 | 62.0 ± 7.4 | 9770 ± 2450 | 842 ± 235 | 1.82 ± 0.49 | 43.5 ± 19.1 | 95.1 ± 13.1 | 332 ± 25.6 |
| 64.9 | 57.3 | 3630 | 505 | 1.00 | 14.9 | 84.7 | 322 | ||
|
| |||||||||
| X 2 | 9.6 (0.02) | 9.7 (0.02) | 6.8 (0.08) | 2.3 (NS) | 13.2 (0.004) | 9.8 (0.02) | 6.0 (NS) | 11.8 (0.008) | |
Table 6.
Contaminant levels in Dolly Varden collected from from the Aleutian Islands during the summer of 2004. Given are means±SE and geometric means below. Comparisons are made with Kruskal-Wallis 1-Way ANOVA, yielding an X2 statistic. All values are in ng/g (ppb wet weight).
| Dolly Varden | Total Length (cm) | Standard Length (cm) | Weight (g) | Arsenic | Cadmium | Lead | Mercury | Selenium | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N | |||||||||
| Amchitka Island | |||||||||
| Fox Lake (Constantine Harbor) | 30 | 27.5 ± 0.97 | 24.4 ± 0.81 | 197 ± 17.5 | 8.01 ± 2.60 | 0.22 ± 0.11 | 17.2 ± 6.30 | 156 ± 24.8 | 135 ± 23.3 |
| 27 | 24.0 | 169 | 0.64 | 0.02 | 0.82 | 0.39 | 32.7 | ||
| Cannikin Lake | 21 | 35.4 ± 1.30 | 32.0 ± 1.13 | 478 ± 47.6 | 17.8 ± 5.10 | 4.50 ± 3.60 | 4.58 ± 2.20 | 158 ± 14.7 | 761 ± 46.8 |
| 34.9 | 31.6 | 435 | 2.85 | 0.17 | 0.36 | 122 | 727 | ||
|
| |||||||||
| Intra-island Kruskal-Wallis | 17.5 (<0.0001) | 20.4 (<0.0001) | 24.8 (<0.0001) | 4.4 (0.04) | 9.9 (0.002) | 0.3 (NS) | 0.6 (NS) | 35.3 (<0.0001) | |
| Amchitka (Totals) | 51 | 30.7 ± 0.96 | 27.5 ± 0.85 | 313 ± 29.3 | 12.0 ± 2.66 | 1.98 ± 1.51 | 12.0 ± 3.88 | 157 ± 15.7 | 393 ± 49.4 |
| 29.9 | 26.9 | 249 | 1.18 | 0.05 | 0.58 | 93.4 | 117 | ||
|
| |||||||||
| Umnak Island | 16 | 38.0 ± 0.96 | 33.4 ± 0.08 | 372 ± 37.7 | 173 ± 27.4 | 0.95 ± 0.42 | 65.2 ± 14.9 | 23.7 ± 5.30 | 292 ± 36.6 |
| 37.8 | 33.3 | 345 | 143 | 0.09 | 17.5 | 17.3 | 258 | ||
| Adak Island | 8 | 32.0 ± 2.74 | 28.6 ± 2.54 | 356 ± 64.7 | 227 ± 44.0 | 4.87 ± 2.74 | 0.08 ± 0.00 | 23.2 ± 8.50 | 188 ± 52.6 |
| 30.8 | 27.5 | 269 | 202 | 0.80 | 0.08 | 5.50 | 80.6 | ||
|
| |||||||||
| X 2 | 34.6 (<0.0001) | 36.7 (<0.0001) | 30.3 (<0.0001) | 51.6 (<0.0001) | 16.2 (0.001) | 22.4 (<0.0001) | 26.1 (<0.0001) | 48.3 (<0.0001) | |
3.3. Interisland differences
Not all fish could be collected on all islands. The flathead sole, the fish with the highest arsenic levels was only collected on Adak. There were significant interisland differences in metal levels for some metals, and some species. Data have been published previously for great sculpin and flathead sole (Burger et al. 2007b) and Pacific cod (Burger et al. 2007c). Tables 2–6 provide intra- and interisland data for species not examined previously. Pink salmon were collected only from one island (Atka), and rock sole had too few samples to examine by island. Black rockfish did not differ in size between Amchitka and Kiska, but there were interisland differences for arsenic and cadmium (slightly higher on Amchitka), and selenium (slightly higher on Kiska)(Table 2). Rock greenling were collected from several islands (Adak, Umnak, Kiska, Amchitka), as well as several locations on Amchitka. There were significant size differences, with Umnak having the longest and heaviest fish as well as the highest levels of arsenic and lead (Table 3).
Yellow Irish lords were also collected at four islands (Table 4). There were no significant interisland differences in length, and only slight differences in weight (p=0.05). Lead was significantly higher on Umnak (p=0.003) and mercury was higher on Kiska (p=0.03). Interisland variation in arsenic and selenium were marginally different (p<0.10) with both being lowest on Adak. Halibut were obtained from Amchitka, Rat, and Kiska Islands, with significant size differences. Amchitka halibut were larger and had non-significantly higher mercury (324 ppb) while Rat Island had high cadmium (32 ppb) and selenium (552 ppb) and Kiska had high lead levels. For the freshwater Dolly Varden all elements shows significant interisland differences but there were no consistent patterns (Table 6). Those from Umnak were significantly larger than those from Adak or Amchitka, and had significantly higher levels only of lead (not usually associated with size), but not mercury. Arsenic and cadmium were highest on Adak.
Overall, the fish examined were at fairly high trophic levels, and represented great variation in age of maturity and lifespan (Table 1, Munk 2001). Metals levels were not generally related to either. That is, black rockfish, with the highest trophic level and longest lifespan, did not have the highest levels of any metal except cadmium. In constrast, rock sole, at the lowest trophic level and an intermediate lifespan, did not have the lowest levels of any metal. The most consistent patterns was that the bottom-dwelling fish had the highest levels of arsenic, as has been previously reported for benthic and flatfish species (De Gieter et al., 2002; Meador et al., 2004), and some of the highest levels of lead, which might also reside in sediments. Even mercury, which normally is highest in top trophic-level predators was not related to trophic level in these fish. However, two of the three species with the highest levels of mercury were the largest species overall. Thus, mercury levels may well relate to both trophic level and relative body size, which in turn reflects how large prey they can eat and the possibility for biomagnification. Within a single species, very large adults may feed at a higher trophic level than average-sized adults.
3.4. Relationship between size and metals levels
For all species there was a significant relationship between length and weight (Table 7). There were some significant correlations between size and metals levels for some species of fish, and some metals. For all species with sufficient samples for analysis, except Dolly Varden, there was a positive relationship between mercury levels and size. The correlation was highest for halibut (that had the greatest size range). There was no consistent size pattern for the other metals. The following significant relationships between size and a metal were found: 1) a positive relationship for cadmium in black rockfish, 2) a positive relationship for lead in rock greenling, 3) a negative relationship for cadmium and a positive relationship of selenium for yellow Irish lord, 4) negative relationships for cadmium and selenium in halibut, and 5) positive associations for arsenic, cadmium and selenium in Dolly Varden. There were no significant correlations of size with metal levels for octopus.
Table 7.
Correlation of size and contaminant levels in 7 species from the Aleutians. Correlations are given for all locations combined. Given are Kendall tau correlations.
| Black Rockfish | Weight | Total Length | Stardard Length | Arsenic | Cadmium | Lead | Mercury | Selenium |
|
| ||||||||
| Weight | ********* | 0.74 (<0.0001) | 0.65 (<0.0001) | NS | NS | NS | 0.39 (0.001) | NS |
| Total Length | ********* | 0.78 (<0.0001) | NS | 0.25 (0.04) | NS | 0.39 (0.002) | NS | |
| Standard Length | ********* | NS | NS | NS | 0.3 (0.01) | NS | ||
| Arsenic | ********* | NS | NS | NS | NS | |||
| Cadmium | ********* | NS | 0.32 (0.009) | NS | ||||
| Lead | ********* | NS | NS | |||||
| Mercury | ********* | NS | ||||||
| Selenium | ********* | |||||||
|
| ||||||||
|
| ||||||||
| Halibut | Weight | Total Length | Stardard Length | Arsenic | Cadmium | Lead | Mercury | Selenium |
|
| ||||||||
| Weight | ********* | 0.88 (<0.0001) | 0.91 (<0.0001) | NS | NS | NS | 0.43 (0.003) | −0.35 (0.02) |
| Total Length | ********* | 0.98 (<0.0001) | NS | −0.28 (0.06) | NS | 0.45 (0.002) | −0.35 (0.02) | |
| Standard Length | ********* | NS | −0.28 (0.06) | NS | 0.47 (0.002) | −0.34 (0.02) | ||
| Arsenic | ********* | NS | NS | NS | NS | |||
| Cadmium | ********* | NS | NS | 0.30 (0.04) | ||||
| Lead | ********* | NS | NS | |||||
| Mercury | ********* | NS | ||||||
| Selenium | ********* | |||||||
|
| ||||||||
|
| ||||||||
| Rock Greenling | Weight | Total Length | Stardard Length | Arsenic | Cadmium | Lead | Mercury | Selenium |
|
| ||||||||
| Weight | ********* | 0.72 (<0.0001) | 0.79 (<0.0001) | NS | NS | 0.25 (0.02) | 0.27 (0.01) | NS |
| Total Length | ********* | 0.82 (<0.0001) | NS | NS | 0.35 (0.002) | 0.21 (0.05) | NS | |
| Standard Length | ********* | NS | NS | 0.32 (0.005) | 0.25 (0.02) | NS | ||
| Arsenic | ********* | NS | NS | NS | NS | |||
| Cadmium | ********* | NS | NS | NS | ||||
| Lead | ********* | NS | NS | |||||
| Mercury | ********* | NS | ||||||
| Selenium | ********* | |||||||
|
| ||||||||
|
| ||||||||
| Yellow Irish Lord | Weight | Total Length | Stardard Length | Arsenic | Cadmium | Lead | Mercury | Selenium |
|
| ||||||||
| Weight | ********* | 0.71 (<0.0001) | 0.73 (<0.0001) | NS | NS | NS | 0.26 (0.02) | 0.22 (0.04) |
| Total Length | ********* | 0.86 (<0.0001) | NS | NS | NS | 0.23 (0.03) | NS | |
| Standard Length | ********* | NS | −0.23 (0.04) | NS | 0.22 (0.04) | NS | ||
| Arsenic | ********* | NS | 0.24 (0.03) | NS | 0.3 (0.005) | |||
| Cadmium | ********* | NS | NS | 0.22 (0.05) | ||||
| Lead | ********* | NS | NS | |||||
| Mercury | ********* | 0.3 (0.003) | ||||||
| Selenium | ********* | |||||||
|
| ||||||||
|
| ||||||||
| Dolly Varden | Weight | Total Length | Stardard Length | Arsenic | Cadmium | Lead | Mercury | Selenium |
|
| ||||||||
| Weight | ********* | 0.70 (<0.0001) | 0.73 (<0.0001) | 0.15 (0.06) | 0.21 (0.02) | NS | NS | 0.37 (<0.0001) |
| Total Length | ********* | 0.87 (<0.0001) | 0.27 (0.001) | NS | NS | NS | 0.31 (0.0001) | |
| Standard Length | ********* | 0.29 (0.0005) | 0.16 (0.07) | NS | NS | 0.33 (<0.0001) | ||
| Arsenic | ********* | 0.22 (0.01) | 0.17 (0.05) | −0.34 (<0.0001) | NS | |||
| Cadmium | ********* | NS | NS | NS | ||||
| Lead | ********* | NS | NS | |||||
| Mercury | ********* | 0.14 (0.07) | ||||||
| Selenium | ********* | |||||||
There were few significant associations among metals in any species and most were only marginally significant (Table 7). P values are listed only for strong associations (p<0.01). We found that 1) mercury was positively associated with cadmium in black rockfish; 2) lead was positively associated with arsenic in yellow Irish lord, 3) selenium was positively associated with arsenic, cadmium, and mercury in yellow Irish lord. 4) selenium was positively associated with cadmium in halibut; 5) arsenic was positively associated with cadmium and lead and negatively associated with mercury in Dolly Varden, and 6) selenium was negatively associated with mercury in Dolly Varden.
4. Discussion
Overall this study found: 1) significant interspecific differences in all metals levels, with no one fish species having the highest levels of any metal at all sites, 2) intraisland locational differences for some species for some metals on Amchitka, 3) significant but inconsistent interisland differences for some species and metals, 4) significant positive size relationship for lead (rock greenling) and mercury (black rockfish, halibut, rock greenling, and yellow Irish lord, and 4) significant negative size relationships for cadmium and selenium in halibut. Although the variations were significant, they were usually not great (except for arsenic, see below). In the following section all results refer to total mercury and have been converted to nanograms per gram (ppb) on a wet weight basis.
4.1. Interspecific differences
There were significant interspecific differences in metal levels for all species. However, the differences were generally within an order of magnitude, except for arsenic. Arsenic is known to be higher in bottom dwelling fish (Meador et al., 2004), and in the present study, levels were 2 orders of magnitude higher for sole than the other species. Both species of sole (rock, flathead) are bottom dwellers, and were expected to have higher levels of arsenic, because very high levels of arsenic in sole and other bottom dwelling flatfish have been noted previously (De Gieter et al., 2002; Meador et al., 2004, Burger et al. 2007b). High and very variable levels were reported in sole from the North Sea with maximum levels of 55,000 ppb in plaice (Pleuronectes platessa), 76,000 ppb in lemon sole (Microstomus kitt) and up to 16,000 ppb in common sole (Solea solea) (De Gieter et al. 2002). Sole live in the silt or mud bottoms from nearshore to less than 366 m in depth, and they mainly stay in contact with the ocean floor (Mecklenburg et al., 2002; Johnson, 2003). Diet also depends upon sediment type (McConnaughey and Smith, 2000). We collected these two species (flathead and rock sole) only from Adak Island, which was occupied by a large military base for a longer duration than occupancy of other islands. Adak is still occupied and is on the EPA’s National Priorities list for its military legacy pollution of several contaminants (ATSDR, 2004).
However, halibut and rock greenling also occur mainly near the bottom and had relatively low levels of arsenic (636 ppb and 381 ppb respectively). The large difference in arsenic levels between species that largely inhabit the benthos requires further study, but may be due to the time spent in each location, food chain differences, or toxicokinetics. It is worth noting, however, that octopus (generally bottom-dwelling) also averaged relatively high levels of arsenic (2160 ppb).
The lack of major interspecific differences in most metals may well be a result of the fish living in marine water that were generally distant from intense industrial, and agricultural and residential development, as is typical of the continental, coastal United States. However, historical military occupation and World War II battles occurred on Adak, Amchitka, and Kiska. Thus, even though the islands were 140 km apart (from Constantine Harbor on Amchitka to Kiska) and 400 km apart (Adak to Kiska), levels were still similar. This was true even through many of the bottom-dwelling fish (sole, rock greenling, black rockfish) are rather sedentary.
4.2. Locational differences
Much of the eastern third of Amchitka had been disturbed and had harbor military during WWII, demolition, and remediation activities that would have been sources of contamination by lead, mercury, cadmium, arsenic. as well as petrochemicals, polyaromatic hydrocarbons, pesticides, and radioactive materials. At Adak, a Naval Air Facility operated on the eastern part of the island from 1942 until 1997(ATSDR, 2004). Over 100,000 military personnel were present at its peak. The Naval station is on the EPA National Priorities List, although not specifically for arsenic (ATSDR, 2004). Presently about 300 people live in the town of Adak, and they still use some of the old military barracks. Some of the military equipment was left on the island, and some was dumped in the harbor. Thus, there is the potential for historical metal and petrochemical contamination from the base operations. ATSDR (2004) identified lead in drinking water (from pipes) as a human hazard, and concluded that there was not a current risk from subsistence consumption of fish and shellfish. However, ATSDR (2004) recommended future seafood monitoring for PCBs, methylmercury, aluminum, arsenic, cadmium, chromium and lead.
Unique geochemical features in marine ecosystems can affect metal bioaccumulation in fish (Meador et al., 2005). There was not a consistent pattern of metals either among species or locations on Amchitka. Halibut, Pacific Cod, and salmon are highly migratory species. We anticipated that their levels would vary little among locations, although after spending a month in a locality, they could reflect local contaminants. Salmon collected on a spawning run probably do not have time to accumulate local metals.
The highest mean arsenic level was in flathead sole (19,500 ppb) collected only on Adak. The relatively high levels of arsenic in fish from Adak bears further examination. Although there were sometimes significant differences between metal levels from fish on Amchitka and Kiska Islands (140 km apart, depending on the site), the differences were not great, and may be largely due to differences in size of the fish from each site.
Mercury, the metal of special concern from a health standpoint (both eco-receptors and humans) did not differ significantly among islands for black rockfish, rock greenling, and halibut, and the difference was only marginally significant for yellow Irish lords. This suggests that there is not a local source of mercury from any of the islands.
Verbrugge (2007) reported results of the Alaska Fish Monitoring Program for mercury samples obtained “around the State”. Only three species were common to our studies. Our mean total mercury results compared with Verbrugge’s are for black rockfish (our results 145 ppb vs 134 ppb), Pacific cod (our results 173 ppb vs 133 ppb) and halibut (our results 158 ppb vs 122 ppb.). Mercury concentration in halibut is size dependent (Verbrugge 2007), and our sample averaged 8.6 kg (ca 19 pounds), corresponding to the lowest size class in Verbrugge (2007). We also compared our mercury results to the data base analysis of Jewett and Duffy (2007) which reported ranges of means for several locations, for several species in common, including pink salmon (our results 40 ppb vs 20–40 ppb), Pacific cod (our results 173 ppb vs 130–150 ppb), rock sole (our results 95 ppb vs 70 ppb) and Dolly Varden (our results 114 ppb vs. 10–250 ppb). For the most part there is agreement between our Aleutian results and the more widespread Alaskan samples.
4.3. Risk to the fish themselves and predators that consume them
Metals in fish can pose a problem to the fish themselves, as well as to predators that consume them. Thus, food chain effects might occur for metals that bioaccumulate. The metal with the highest relative concentrations was arsenic. Although documented arsenic poisoning is relatively rare in wildlife, very high levels can be toxic (Eisler, 1994). Most arsenic in fish occurs in organic forms (particularly arsenobetaine and arsenocholine), which are less toxic than the inorganic species (Eisler, 1994; ATSDR, 2000). “Toxic” arsenic species include inorganic arsenites (As-III) and arsenates (As-V), as well as organic metabolites monomethylarsonic (MMA) and dimethylarsinic acids (DMA). De Gieter et al. (2002) found that toxic arsenic (median about 200 ppb from graph) comprised about 0.5% of total arsenic in lemon sole. Peshut et al. (2008) reported that most of the Samoan Reef fish had less than 0.5% of the total arsenic in inorganic form, although shellfish ranged from 1–5% inorganic. Residues of arsenic over 10,000 ppb in liver or kidney of birds indicate arsenic poisoning (Goede, 1985), and levels of 5,000 to 10,000 ppb are indicative of arsenic poisoning in domestic animals (Vreman et al., 1986). Thus the mean levels of arsenic found in most fish from the Aleutians (means of less than 1,500 ppb) are well below this level, suggesting no effect on the fish themselves or their consumers. However, the levels for yellow Irish lord (mean of 4,340 ppb) and flathead sole (mean of 19,500) could be toxic to birds and bear further examination.
Cadmium is a teratogen, carcinogen, and a possible mutagen, and in fish it can cause anemia, osmoregulatory problems, decreased digestive efficiency, biochemical effects, and erratic swimming (Larsson, 1977; Reid and McDonald, 1988; Haux and Larsson, 1984; Eisler, 1985). In laboratory experiments, Handy (1993) found that cadmium levels of 70 to 340 ppb in fish muscle can cause death. In this study, mean cadmium concentrations in muscle did not exceed 18 ppb, suggesting that there is no cause for concern for consumers of these fish. However, fish themselves are affected by chronic cadmium exposure to the eggs or fry (Prager, 1995; Hansen et al., 2002). There is a great deal of variation in susceptibility among fish species; salmonids are more sensitive than other species (Wren et al., 1995). Whole body burdens of cadmium in fish in the United States average about 30 ppb (wet weight, Schmitt and Brumbaugh, 1990). The levels for fish from the Aleutians are similar or slightly lower. Adverse effects of cadmium in fish and wildlife can occur when cadmium levels exceed 3 ppb in freshwater, 4.5 in saltwater, and 1,000 ppb in the diet (Eisler, 1985). Thus, our sampling did not find cadmium levels that pose a threat to the fish themselves or to wildlife that consume them.
Lead is a nephro- and neurotoxin that produces behavior deficits, and decreases in survival, growth, learning, and metabolism (Weber and Dingel, 1997; Eisler, 1988; Burger and Gochfeld, 2000). For fish, lead is also a neurotoxin that can cause behavioral deficits within days of exposure (Weber and Dingel, 1997), as well as increased mucus formation (Eisler, 1988). Levels of 50,000 ppb in the diet may produce adverse effects in some predators, but levels as low as 100 ppb to 500 ppb are associated with learning deficits in some sensitive vertebrates (Eisler, 1988). In the present study, mean lead levels were 70 ppb, or lower, suggesting little cause for concern for either the fish themselves, or their predators.
Mercury is the usual contaminant of concern in marine ecosystems because it is both natural occurring, and comes from anthropogenic sources. In some fish, mercury levels of 5000 ppb (wet weight) can cause emaciation, loss of appetite, decreased coordination, and mortality, but in others, concentrations of 15,000 ppb are necessary to cause ill effects (Eisler, 1987; Wiener and Spry, 1996). Sensitive birds, however, can experience adverse effects at dietary concentrations of 50 to 500 ppb. In the present study, mercury concentrations averaged less than 300 ppb, suggesting no effects on the fish themselves, but possible effects on birds that prey on some fish.
Selenium is an essential micronutrient that can be toxic at high levels (Coyle et al., 1993; Ohlendorf et al., 1995; Ohlendorf, 2000). Concentrations of 1000 ppb in food are toxic to other wildlife that consume them, while concentrations of 2,600 ppb are associated with adverse effects in the fish themselves (Lemly, 1993a,b). The levels of selenium found in the Aleutian fish averaged below 625 ppb, suggesting little cause for concern for either the fish themselves, or for predators that consume them.
Thus, with the exception of arsenic, the average levels of metals in the Aleutian fish examined were not high enough to cause concern either for the fish themselves, or for predators that consume them. However, the mean levels of arsenic in yellow Irish lord and flathead sole bear further examination because they are above the known effects levels. Flathead sole is eaten by Aleuts, suggesting that further examination of consumption patterns and rates is essential (see below).
4.4. Risk to human consumers
There is an on-going national discussion about the benefits and risks of consuming fish, including species, amounts, and size of the fish involved. Much of this discussion focuses on recreational and subsistence fish, and subsistence fish is certainly an issue for residents on the Aleutians (Patrick, 2002; Fall et al., 2006). Egeland and Middaugh (1997) urged that the benefits of native/natural diets high in fish consumption outweighed any mercury risk, and this is reflected in the Alaska fish fact sheet (Alaska 2007). The Aleuts live in relatively small villages in the Aleutians, and are reliant on subsistence foods because commercial food has to be brought in by airplane (which runs once or twice a week in good weather) or by boat (3–4 times a year depending upon weather). In either case, the amount of food that can be brought in is limited, and it is expensive. Thus, the people rely on and prefer local resources, mainly fish (APIA, 2002; Hamrick and Smith, 2003), and fish comprise about 60% of subsistence harvests in Alaska (Jewett and Duffy 2007). Depending upon the village, 30–90 % of the foods eaten by Aleuts are subsistence foods (Patrick, 2002). Many of the fish collected for this paper were caught by Aleut member of our expedition, in the traditional manner. The fish collected by Aleut fishermen were similar in size to those collected by the other scientists on the expedition (Burger et al. 2006). Equally important, however, is the fact that half or more of the fish sold in the continental United States comes from the Bering Sea; cod and halibut are important commercial fish sold in markets throughout the U.S. (AFSC, 2003). Most metals are well below the levels known to affect vertebrates; arsenic and mercury are exceptions.
Arsenic, like mercury, is difficult to examine because levels are usually measured as total arsenic (as was done in this study), but there are differences in effects depending upon the chemical species. For mercury, it is the organic form (methylmercury) that is most toxic to vertebrates, whereas for arsenic, it is the inorganic forms that are most toxic to humans. Inorganic arsenic can cross the placenta and cause effects on fetuses in mammals (Nagymajtenyi et al. 1985). The U.S. Environmental Protection Agency sets human health protection criterion of > 1.3 mg/kg (= 1,300 ppb) fresh weight in fish tissue (Eisler 1994). In this study the mean levels of arsenic fell into three categories: 1) low levels (69.3 ppb in Dolly Varden) to 636 ppb in Pacific halibut), 2) intermediate (1,400 ppb in great sculpin, 2,180 ppb in Pacific cod, and 4,340 ppb in rock sole), 3) high (19,500 ppb in fladhead sole).
Using the estimate of 0.5% of total arsenic as “toxic” (De Gieter et al. 2002) or “inorganic” (Peshut et al. 2008), the 19,500 ppb (ng/g) in flathead sole translates into 97.5 ng/g (inorganic As) or or 22.1 micrograms/8 ounce (228g) meal or 0.3 micrograms/kg for a 70 kg person per meal. A person eating one such meal/day would ingest exactly the EPA Reference Dose of 0.0003 mg/kg/day (USEPA, 2007). The conversion from total to inorganic is very inexact. Although the Reference Dose calculations includes uncertainty factors, the observed levels indicate that some consumers might reach toxic levels of arsenic from consuming certain Aleutian fish.
Methylmercury is the contaminant in fish that usually poses the greatest risk to humans. Understanding the relationship between fish size and metal levels is important because it has implications for effects on the fish themselves, and on consumers of fish, such as humans. In general, mercury levels increase with fish size (Braune, 1987; Lange et al., 1994; Burger and Gochfeld, 2012), which also occurred in the Aleutian fish. The highest levels of mercury were in flathead sole, yellow Irish lord, and great sculpin, with mean levels close to 0.3 ppm. Many individual fish of these species had levels above 0.3 ppm. The FDA action level is 1 ppm (USFDA 2001, 2003, 2005, 2011), but many states and countries have levels of 0.3–0.5 ppm as their standard. Our data thus suggest that for these three species, considerable attention needs to be devoted to consumption patterns, especially for Aleut subsistence peoples that are dependent on local resources. The sculpins are not preferred fish (although we ate them). However, on Adak especially, flathead sole can be caught easily from the docks without needing to go offshore and are eaten by residents. Even at a mean mercury concentration of 0.15 ppm (exceeded by halibut and cod in our sample), a daily 170 g (6 ounce meal) would expose a 70 kg person to 0.36 micrograms/kg/day. This exceeds the ATSDR MRL (0.3 μg/kg/day) as well as the EPA RfD (0.1 μg/kg/day).
The State of Alaska Department of Health and Social Services has issued a fish fact sheet (Alaska 2007) “Fish Facts and Consumption Guidelines”. which recommends that people “Eat fish at least twice a week,” and that “Women who are or can become pregnant, nursing mothers, and children aged 12 years and under should continue unrestricted consumption of fish from Alaska waters that are low in mercury, which include all five species of Alaska salmon, Pacific cod, walleye pollock, black rockfish, Pacific ocean perch, halibut under 20 pounds, and lingcod <30 inches (Alaska 2007 based on Verbrugge 2007). Arnold and Middaugh (2004) articulated four reasons that federal fish consumption guidelines are not applicable to Alaska, including: 1) methylmercury concentrations are very low in the most frequently consumed Alaskan fish, 2) research has documented numerous benefits, 3) subsistence lifestyle and diet are of great importance to Alaska Natives, and 4) “known benefits of fish consumption far outweigh the theoretical and controversial potential adverse health effects.” Although we find Arnold and Middaugh (2004) overly dismissive of the risks, our data on mercury in these ten fish species from the Aleutians found no species with mean mercury levels above 0.3 ppm (300 ppb). However, sole are one of the fish types mentioned by Arnold and Middaugh (2004) as frequently consumed, and the risks may arise from arsenic, rather than mercury.
Figure 2.
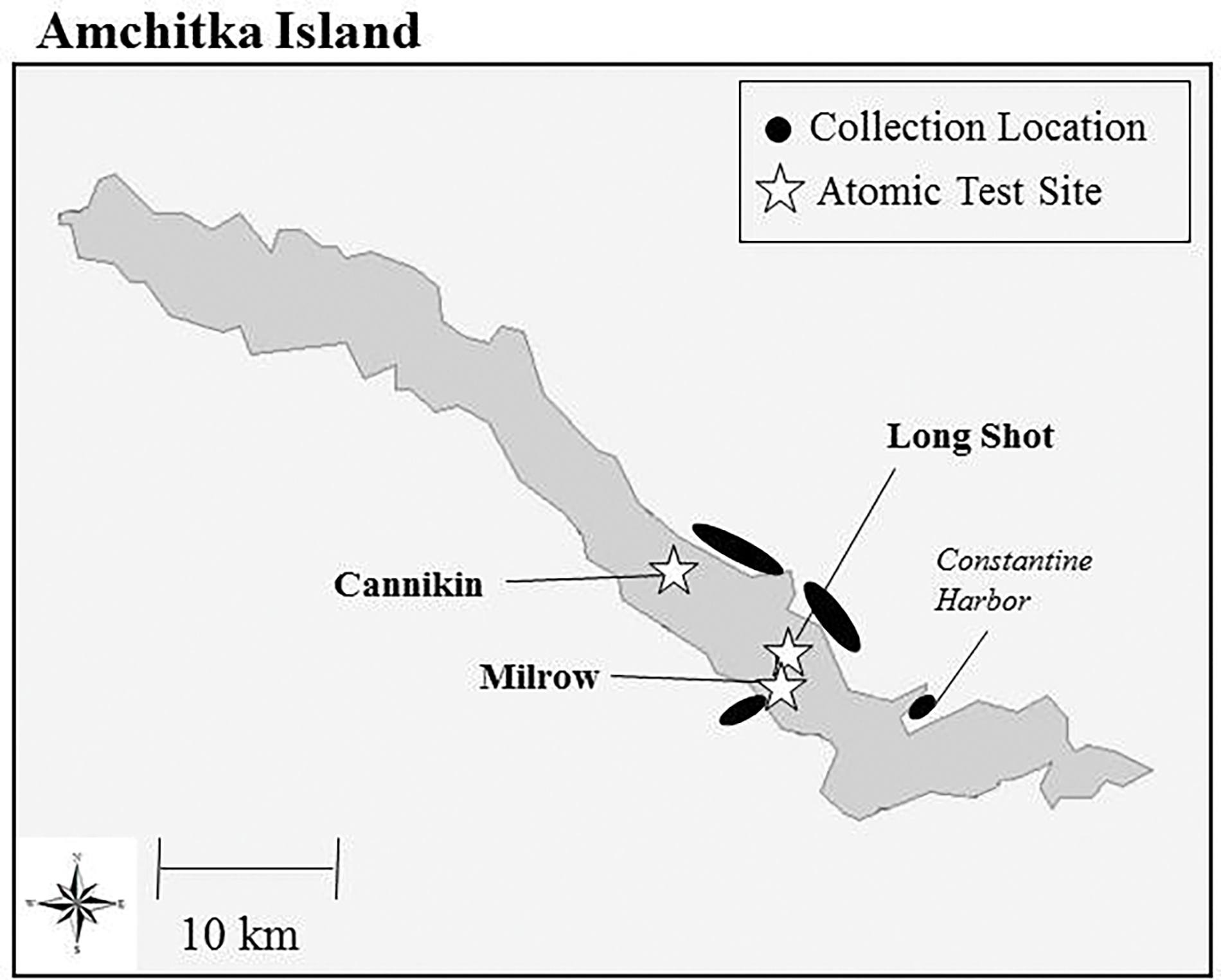
Map of Amchitka Island identifying the three underground nuclear test sites, and the adjacent coastal areas where fish were collected to assess possible contamination from the tests.
Acknowledgements
Fish were collected under appropriate state permits, and our studies were approved by the Rutgers University Animal Review Board. We thank the many people who contributed to the development and execution of CRESP’s Amchitka Geophysical and Biological Project, especially C. W. Powers, D. Kosson, B, Friedlander, C. Jeitner, S. Burke, D. Volz, and S. Jewett. We also thank the following for help throughout the project, D. Barnes, L. Duffy, A. Morkill, R. Patrick, D. Rogers, D. Dasher, and the people of the villages of Unalaska, Nikolski, Atka, and Adak in the Aleutians. We thank the entire crew of the Ocean Explorer, Captain Ray Haddon, mate Glenn Jahnke, cook Don Dela Cruz, and Bill Dixon, Joao Do Mar, and Walter Pestka, for making our field work possible and pleasant, and for bringing us safely back to port. This research was funded by the Consortium for Risk Evaluation with Stakeholder Participation (CRESP) through the Department of Energy (DE-FG 26-00NT 40938, DE-FC01-86EW07053), the Division of Life Sciences of Rutgers University, by Wildlife Trust, and by NIEHS P30ES005022. The results, conclusions and interpretations reported herein are the sole responsibility of the authors, and should not in any way be interpreted as representing the views of the funding agencies.
Funding Sources
This research was conducted under a Rutgers University Animal Review Board Protocol E#97-017), and under appropriate collecting permits (Alaska’s Department of Fish and Game permit # CF-04-043). This work was funded by (DOE Department of Energy, DE-FC01-86EW07053), and we have no conflict of interest with respect to the research or funding.
References
- Agency for Toxic Substances and Disease Registry (ATSDR) 2000. Toxicological Profile for Arsenic. US Agency for Toxic Substances and Disease Registry, Atlanta, GA. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) 2004. Public Health Assessment: Naval Air Facility, Adak.http://www.atsdr.cdc.gov/HAC/PHA/adak/ada_p1.html (accessed: 8.9.2013)
- Alaska. (2007) Alaska 2007 Fish Facts and Consumption Guidelines. (Accessed September 2, 2013) http://www.epi.alaska.gov/eh/fish/
- Alaska Fisheries Science Center (AFSC) 2003. Alaska fisheries. (Accessed June 1, 2013) http://www/afsc/noaa.gov/species/Pollock.htm
- Aleutian Pribilof Islands Association (APIA) 2002. Atka traditional food sampling survey. In: Graham JD, Wiener JB (Eds.), Risk versus Risk: Tradeoffs in Protecting Health and the Environment. Harvard University Press, Cambridge, MA, pp 129–157. [Google Scholar]
- Arnold SM, Middaugh JP 2004. Use of Traditional Foods in a Healthy Diet in Alaska: Risks in Perspective. Vol. 2: Mercury Department of Health and Social Services Anchorage, Alaska [Google Scholar]
- Bienenfeld LS, Golden AL, Garland EJ, 2003. Consumption of fish from polluted waters by WIC participants in East Harlem. J. Urban Health 80, 349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braune BM, 1987. Mercury accumulation in relation to size and age of Atlantic Herring (Clupea harengus harengus) from the Southwestern Bay of Fundy, Canada. Arch. Environ. Contam. Toxicol. 16, 311–320. [DOI] [PubMed] [Google Scholar]
- Brunner EJ, Jones PJS, Friel S, Bartley M, 2009. Fish, human health and marine ecosystem health: policies in collision. Intl. J. Epidemiol. 38, 91–100. [DOI] [PubMed] [Google Scholar]
- Buck NJ, Gobler CJ, Sanudo-Wilhelmy SA, 2005. Dissolved trace element concentrations in the east River-Long Island Sound system: relative importance of autochthonous versus allochthonous sources. Environ. Sci. Techno.l 39, 3528–3537. [DOI] [PubMed] [Google Scholar]
- Burger J, 2000. Consumption advisories and compliance: the fishing public and the deamplification of risk. J. Environ. Plan. Manage. 43, 471–488. [Google Scholar]
- Burger J, 2002. Consumption patterns and why people fish. Environ. Res. 90, 125–35. [DOI] [PubMed] [Google Scholar]
- Burger J, 2006. Bioindicators: types, development, and use in ecological assessment and research. Environ. Bioindic 1, 1–18. [Google Scholar]
- Burger J, Gochfeld M, 1991. Cadmium and lead in common terns (Aves: Sterna hirundo): Relationship between levels in parents and eggs. Environ. Res. 16, 253–258. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, 2007. Risk to consumers from mercury in Pacific cod (Gadus macrocephalus) from the Aleutians: fish age and size effects. Environ. Res. 105, 276–84. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, 2011. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci. Total Environ. 409, 1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Gochfeld M, 2001a. Ethnic differences in risk from mercury among Savannah River fishermen. Risk Anal. 21, 533–44. [DOI] [PubMed] [Google Scholar]
- Burger J, Gaines KF, Boring CS, Stephens WL Jr, Snodgrass J, Gochfeld M, 2001b. Mercury and selenium in fish from the Savannah River: species, trophic level, and locational differences. Environ. Res 87, 108–18. [DOI] [PubMed] [Google Scholar]
- Burger J, Fleischer J, Gochfeld M, 2003. Fish, shellfish, and meat meals of the public in Singapore. Environ. Res. 93, 254–61. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Burke S, Jeitner CW, Jewett S, Snigaroff D, Snigaroff R, Stamm T, Harper S, Hoberg M, Chenelot H, Patrick R, Volz CD, Weston J 2006. Do scientists and fishermen collect the same size fish? Possible implications for exposure assessment. Environ. Res. 101:34–41. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T, Snigaroff R, Snigaroff E, Patrick D, Weston J, 2007a. Mercury levels and potential risk from subsistence foods from the Aleutians. Sci. Tot. Environ. 384, 93–105. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Jeitner C, Burke S, Stamm T, 2007b. Metal levels in flathead sole (Hippoglossoides elassodon) and great sculpin (Myoxocephalus polyacanthocephalus) from Adak Island, Alaska: potential risk to predators and fishermen. Environ Res 103, 62–9. [DOI] [PubMed] [Google Scholar]
- Burger J, Gochfeld M, Shukla T, Jeitner C, Burke S, Donio M, Shukla S, Snigaroff R, Snigaroff E, Stamm T, Volz C, 2007c. Heavy metals in Pacific cod (Gadus macrocephalus) from the Aleutians: location, age, size, and risk. J. Toxicol. Environ. Health 70, 1897–1911. [DOI] [PubMed] [Google Scholar]
- Canada Agriculture and Agri-Food (CAAF). 2013. Consumer trends: The American seafood market. Canada, CAAF. [Google Scholar]
- Choi AL, Budtz-Jorgensen E, Jorgensen PJ, Salonen JT, Tuomainen T, Murata K, Nielsen HP, Petersen MS, Askham J, Grandjean P, 2009. Methylmercury exposure and adverse cardiovascular effects in Faroese whaling men. Environ. Health Perspect. 117, 367–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen DM, Heifetz J 2002. The Northern rockfish, Sebastes polyspinis, in Alaska: commercial fishery, distribution, and biology. Mar. Fish. Rev. 64, 1–27. [Google Scholar]
- CRESP 2005. Amchitka Independent Science Assessment: Biological and Geophysical Aspects of Potential Radionuclide Exposure in the Amchitka Marine Environment. (Accessed August 9, 2013) http://www.cresp.org/Amchitka/Amchitka_Final_Report/index_FinalReport.html
- Coyle JJ, Ingersoll DR, Fairchild CG and May TW 1993. Effects of dietary selenium on the reproductive success of bluegills (Lepomis macrochirus). Environ. Toxicol. Chem. 12, 551–565. [Google Scholar]
- De Gieter MD, Leermakers M, Ryssen RV, Noyen J, Goeyens L, Baeyens W 2002. Total and toxic arsenic levels in North Sea fish. Arch. Environ. Contam. Toxicol. 43, 406–417. [DOI] [PubMed] [Google Scholar]
- Dorea JG, Moreira MB, East G, Barbosa AC 1998. Selenium and mercury concentrations in some fish species of the Madeira River, Amazon Basin, Brazil. Biol. Trace Elem. Res. 65, 211–220. [DOI] [PubMed] [Google Scholar]
- Egeland GM, Middaugh JP, 1997. Balancing fish consumption benefits with mercury exposure. Science. 278:1904–1905. [DOI] [PubMed] [Google Scholar]
- Eisler R, 1985. Cadmium hazards to fish, wildlife and invertebrates: a synoptic review. U.S. Fish & Wildlife Service, Biological Report 85 (1.2). Washington D.C. [Google Scholar]
- Eisler R, 1987. Mercury hazards to fish, wildlife and invertebrates: a synoptic review. U. S. Fish & Wildlife Service, Biological Report 85 (1.10) Washington DC [Google Scholar]
- Eisler R, 1988. Lead hazards to fish, wildlife, and invertebrates: A synoptic review. U. S. Fish and Wildlife Service, Washington, DC. [Google Scholar]
- Eisler R, 1994. A review of arsenic hazards to plants and animals with emphasis on fishery and wildlife resources. In: Nriagu JO (Ed.) Arsenic in the Environment, Part II John Wiley, New York, NY. Pp 185–259. [Google Scholar]
- Fall JA, Stanek RT, Brown L, 2006. Utermohle C. The harvest and use of fish, wildlife and plant resources in False Pass, Unimak Island, Alaska. Alaska Dept. of Fish & Game, Paper No. 183, Juneau, AK; (Accessed June 6, 2013). http://nativeknowlege.org/db/files/tp183.htm, [Google Scholar]
- Fishwatch. 2013. The surprising sources of your favorite seafoods. (Accessed August 1, 2013). http://www.fishwatch.gov/features/top10seafoods_and_sources_10_10_12.html.
- Fitzgerald WF, 1989. Atmospheric and oceanic cycling of mercury. In: Riley JP, Chester R (Eds.). Chemical oceanography, Vol. 10, Academic Press, New York, pp. 151–186 [Google Scholar]
- Fitzgerald WF, Engstrom DR, Lamborg CH, Tseng CM, Balcom PH, Hammerschmidt CR, 2005. Modern and historic atmospheric mercury fluxes in northern Alaska: global sources and Arctic depletion. Environ. Sci. Technol. 39, 557–568. [DOI] [PubMed] [Google Scholar]
- Freire C, Ramos R, Lopez-Expinosa M, Diez S, Vioque J, Ballester F, Fernandez M, 2010. Hair mercury levels, fish consumption, and cognitive development in preschool children from Granada, Spain. Environ. Res. 110, 96–104. [DOI] [PubMed] [Google Scholar]
- Gochfeld M, 2003. Cases of mercury exposure, bioavailability, and absorption. Ecotoxicol. Environ. Safety 56, 174–179. [DOI] [PubMed] [Google Scholar]
- Gochfeld M, Burger J, 2005. Good fish/bad fish: a composite benefit-risk by dose curve. Neurotoxicol. 26, 511–520. [DOI] [PubMed] [Google Scholar]
- Goede AA 1985,. Mercury, selenium, arsenic and zinc in waders from the Dutch Wadden Sea. Environ. Pollut. 37A, 287–309. [Google Scholar]
- Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorenson N, Jorgensen PJ 1997. Cognitive deficit in 7-year old children with prenatal exposure to methylmercury. Neurotoxicol. Teratol. 19, 418–28. [DOI] [PubMed] [Google Scholar]
- Guallar E, Sanz-Gallardo MI, van’t Veer P, Bode P, Aro A, Gomez-Aracena J 2002. Heavy metals and myocardial infarction study group: mercury, fish oils, and the risk of myocardial infarction. N. Engl. J. Med. 347, 1747–1754. [DOI] [PubMed] [Google Scholar]
- Hamrick K, Smith J 2003. Subsistence food use in Unalaska and Nikolski’, Aleutian Pribilof Island Association, Anchorage, AK. [Google Scholar]
- Handy RD, 1993. The effect of acute exposure to dietary Cd and Cu on organ toxicant concentrations in rainbow trout, Oncorhynchus mykiss. Aquat. Toxicol. 21, 1–14. [Google Scholar]
- Hansen JA, Welsh PG, Lipton J, Suedkamp MJ 2002. The effects of long-term cadmium exposure on the growth and survival of juvenile bull trout (Salvelinus confluentus). Aquat. Toxicol. 58, 165–174. [DOI] [PubMed] [Google Scholar]
- Harris SG, Harper BL,1998. Native American exposure scenarios and a tribal risk model. Risk. Anal. 17, 789–795. [DOI] [PubMed] [Google Scholar]
- Harris SG, Harper BL, 2000. Using eco-cultural dependency webs in risk assessment and characterization of risks to tribal health and cultures. Environ. Sci. Pollut. Res. 2, 91–100. [Google Scholar]
- Haux C, Larsson A, 1984. Long-term sublethal physiological effects on rainbow trout, Salmo gairdneri, during exposure to cadmium and after subsequent recovery. Aquat. Toxicol. 5, 129–142. [Google Scholar]
- Hightower JM, Moore D, 2003. Mercury levels in high-end consumers of fish. Environ. Health Perspect. 111, 604–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA, Foran JA, Carpenter DO, Hamilton MC, Knuth BA, Schwager SJ, 2004. Global assessment of organic contaminants in farmed salmon. Sci. 303, 226–229. [DOI] [PubMed] [Google Scholar]
- Hsiao H, Ullrich SM, Tanton TW, 2011. Burdens of mercury in residents of Temirtau, Kazakhstan. 1: hair mercury concentrations and factors of elevated hair mercury levels. Sci. Total Environ. 409, 2272–80. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (IOM), 1991. Seafood Safety. National Academy Press, Washington, DC. 432 pp. [Google Scholar]
- Institute of Medicine (IOM), 2006. Seafood Choices: Balancing benefits and risks. National Academy Press, Washington, DC. 722 pp. [Google Scholar]
- Jewett SC, Duffy LK,2007. Mercury in fishes of Alaska, with emphasis on subsistence species. Sci Total Environ. 387, 3–27. [DOI] [PubMed] [Google Scholar]
- Johnson T 2003. The Bering Sea and Aleutian Islands. Alaska Sea Grant College Program, SG-Ed-42, University of AL, Fairbanks. [Google Scholar]
- Lange TR, Royals HE, Connor LL, 1994. Mercury accumulation in largemouth bass (Micropterus salmoides) in a Florida lake. Environ. Contam. Toxicol. l 27, 466–471. [DOI] [PubMed] [Google Scholar]
- Larsson A, 1977. Some experimentally induced biochemical effects of cadmium on fish from the Baltic Sea. Ambio Spec. Rep. 5, 1–67. [Google Scholar]
- Lemly DA, 1993a. Guidelines for evaluating selenium data from aquatic monitoring and assessment studies. Environ. Monit. Assess. 28, 83–100. [DOI] [PubMed] [Google Scholar]
- Lemly DA, 1993b. Metabolic stress during winter increases the toxicity of selenium in fish. Aquat. Toxicol. 27, 133–158. [Google Scholar]
- Li XY, Wang YG, Luo GP, Chen X, Yang XL et al. , 2012. Integrated assessment of heavy metal contamination in sediments from a coastal industrial basin, NE China. PLoSOne 7(6)e39690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YZ, Yan BX, Wang MJ, Guo LY, 2008. The evolution rule and ecology risk assessment of mercury in fish of Gonghua River. J. Agro-Environ. Sci. 27, 2430–2433. [Google Scholar]
- McConnaughey RA, Smith KR, 2000. Associations between flatfish abundance and surficial sediments in the eastern Bering Sea. Can. J. Fish. Aquat. Sci. 57, 2410–2419. [Google Scholar]
- Meador JP, Robisch PA, Clarke RC Jr., Ernest D 1998. Elements in fish and sediment from the Pacific Coast of the United States: Results from the National Benthic Surveillance Project. Mar. Poll. Bull. 37, 56–66. [Google Scholar]
- Meador JP, Ernest D, Kagley A 2004. Bioaccumulation of arsenic in marine fish and invertebrates from Alaska and California. Archiv. Enviorn. Contam. Toxicol. 47, 223–233. [DOI] [PubMed] [Google Scholar]
- Meador JP, Ernest DW, Kagley AN, 2005. A comparison of the non-essential elements cadmium, mercury, and lead found in fish and sediment from Alaska and California. Sci. Total Environ. 339, 189–205. [DOI] [PubMed] [Google Scholar]
- Mecklenburg CW, Mecklenburg TA, Thornsteinson LK 2002. Fishes of Alaska, American Fisheries Society, Bethesda, MD. 1037 pp. [Google Scholar]
- Munk KM, 2001. Maximum age of groundfishes in waters off Alaska and British Columbia and considerations of age determination. Alaska Fish. Res. Bull. 8, 12–21. [Google Scholar]
- Nagymajtenyi L, Selpes A, Berencsi G, 1985. Chromosomal aberrations and fetotoxic effects of atmospheric arsenic exposure in mice. J. Appl. Toxicol. 5, 61–63. [DOI] [PubMed] [Google Scholar]
- National Research Council (NRC),1996. The Bering Sea ecosystem. National Academy Press, Washington, D.C. 308 pp. [Google Scholar]
- National Research Council (NRC), 2000. Toxicological effects of methylmercury. National Academy Press, Washington, D.C. 344 pp. [Google Scholar]
- National Oceanographic and Atmospheric Administration (NOAA). 2010. Pacific Cod. National Marine Fisheries Service. (Accessed July 2, 2013) http:www/afsc/noaa.gov/species/Pacific_cod.
- National Oceanographic and Atmospheric Administration (NOAA). 2012. Communities: the U.S. population living in coastal watershed counties. (Accessed April 3, 2013) http://stateofthecoast.noaa.gov/population/welcome.html.
- National Oceanographic and Atmospheric Administration (NOAA). 2013. Protecting, conserving, managing marine resources in Alaska. (Accessed July 1, 2013). http://www.afsc.noaa.gov/race/behavioral/halibut_fbe.htm
- Nygard T, Lie E, Roy N, Steinnes E, 2001. Metal dynamics in an Antarctic food chain. Mar. Pollut. Bull. 42, 598–602. [DOI] [PubMed] [Google Scholar]
- Ohlendorf HM, 2000. Ecotoxicology of selenium. In: Hoffman DJ, Rattner BA, Burton GS Jr., Cairns J Jr. (Eds). Handbook of ecotoxicology. Lewis Publ., Boca Raton, FL., pp 465–500. [Google Scholar]
- Ohlendorf HM, Hothem RL, 1995. Agricultural drainage effects on wildlife in Central California. In: Hoffman DJ, Rattner BA, Burgon GS Jr, Cairns J Jr. (Eds). Handbook of ecotoxicology, Lewis Publ, Boca Raton, FL., pp. 577–585. [Google Scholar]
- Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW, 2008. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am. J. Epidemiol. 167, 1171–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick R, 2002. How local Alaska native communities view the Amchitka issue. In Proceedings of the Amchitka Long-term Stewardship Workshop. CRESP/University of Alaska, Fairbanks, AK. [Google Scholar]
- Patterson J, 2002. Introduction: comparative dietary risk: balance the risks and benefits of fish consumption. Comments Toxicol. 8, 337–44. [Google Scholar]
- Peshut PJ,Morrison RJ, Brooks BA, 2008. Arsenic speciation in marine fish and shellfish from American Samoa. Chemosph. 71:484–92. [DOI] [PubMed] [Google Scholar]
- Pinheiro MCN, de Nascimento JLM, Silveira LCL, daRocha JBT, Aschner M 2009. Mercury and selenium – a review on aspects related to the health of human populations in the Amazon. Environ. Bioindic. 4, 222–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers CW, Burger J, Kosson D, Gochfeld M, Barnes D, (Eds). 2005. Biological and Geophysical Aspects of Potential Radionuclide Exposure in the Amchitka Marine Environment. CRESP, Piscataway, New Jersey: (available at www.cresp.org). [Google Scholar]
- Prager JC, 1995. Environmental contaminant reference databook, vol. 1. Van Nostrand Reinhold, New York, NY. 1312 pp. [Google Scholar]
- Ramel A, Martinez JA, Kiely M, Bandarra NM, Thorsdottir I, 2010. Moderate consumption of fatty fish reduces diastolic blood pressure in overweight and obese European young adults during energy restriction. Nutrit. 26, 168–74. [DOI] [PubMed] [Google Scholar]
- Reid SD, McDonald DG, 1988. Effects of cadmium, copper, and low pH on ion fluxes in the rainbow trout, Salmo gairdneri. Can. J. Fish Aquat. Sci. 45, 244–253. [Google Scholar]
- Rice G, Swartout J, Mahaffey K, Schoeny R, 2000. Derivation of U.S. EPS’s oral Reference Dose (RfD) for methylmercury. Drug Chem. Toxicol. 23, 41–54. [DOI] [PubMed] [Google Scholar]
- Statistical Analysis System (SAS). 2005. SAS users’ guide. SAS Institute, Cary, North Carolina. [Google Scholar]
- Schmitt CJ, Brumbaugh WG, 1990. National contaminant biomonitoring program: concentrations of arsenic, cadmium, copper, lead, mercury, selenium and zinc in U.S. Freshwater fish, 1976–1984. Arch. Environ. Contam. Toxicol. 19, 731–747. [DOI] [PubMed] [Google Scholar]
- Takezaki T, Inoue M, Kataoka H, Ikeda S, Yoshida M, Ohashi Y, Tajima K, Tominaga S, 2003. Diet and lung cancer risk from a 14-year population-based prospective study in Japan: with special reference to fish consumption. Nutrit. Cancer 45, 160–7. [DOI] [PubMed] [Google Scholar]
- Toth JF Jr.,, Brown RB, 1997. Racial and gender meanings of why people participate in recreational fishing. Leisure Sci. 19:129–46. [Google Scholar]
- US Environmental Protection Agency (USEPA). 2007. Integrated Risk Information System— Arsenic, inorganic (CASRN 7440-38-2). (Accessed August 19, 2013) http://www.epa.gov/iris/subst/0278.htm
- US Food and Drug Administration (USFDA) 2001. FDA consumer advisory.). (Accessed August 19, 2013) http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. Washington, DC: U.S. Food and Drug Administration [Google Scholar]
- US Food and Drug Administration (USFDA). 2003. FDA consumer advisory.Washington, DC: U.S. Food and Drug Administration. (Accessed January 14, 2013) http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. [Google Scholar]
- US Food and Drug Administration (USFDA). 2005. Mercury Levels in Commercial Fish and Shellfish. Washington, DC, Food and Drug Administration. Washington, D.C.: U.S. Food and Drug Administration; (Accessed May 23, 2013). http//www.fda.gov/bbs/topics/ANSWERS/2000/advisory.html. [Google Scholar]
- US Food and Drug Administration (USFDA)(2011) 1991–2004 total diet study – analytical results. (Accessed April 3, 2013) http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration?TotalDietStudy/ucm184293.htm
- Verbrugge LA 2007. Fish consumption advice for Alaskans: A risk management strategy to optimize the public’s health. Alaska Dept of Health and Social Services, Juneau. [Google Scholar]
- Virtanen JK, Mozaffarian D, Chiuve SE, Rimm EB, 2008. Fish consumption and risk of major chronic disease in men. Am. J. Clin. Nutrit. 88, 1618–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreman K, Veen NG, Molen EJ, deRuig WB, 1986. Transfer of cadmium, lead, mercury and arsenic from feed into milk and various tissues of dairy cows: chemical and pathological data. Neth J. Agric. 34, 129–144. [Google Scholar]
- Webe DN., Dinge WM., 1997. Alterations in neurobehavioral responses in fishes exposed to lead and lead-chelating agents. Am. Zool. 37, 354–362. [Google Scholar]
- Wiener JG, Spry DJ,. 1996. Toxicological significance of mercury in freshwater fish. In: Beyer WN, Heinz GH, Redmon-Norwood AW (Eds.) Environmental Contaminants in Wildlife: Interpreting Tissue Concentrations. Lewis Publishers, Boca Raton, FL. pp, 409–463. [Google Scholar]
- World Health Organization (WHO). 1989. Mercury-environmental aspects. WHO, Geneva, Switzerland. [Google Scholar]
- Wren CD, Harris S, Harttrup N, 1995. Ecotoxicology of mercury and cadmium. In: Hoffman DJ, Rattner BA, Burton GA Jr,, Cairns J Jr,(Eds.) Handbook of Ecotoxicology Lewis Publishers; Boca Raton, FL. pp. 392–423. [Google Scholar]


