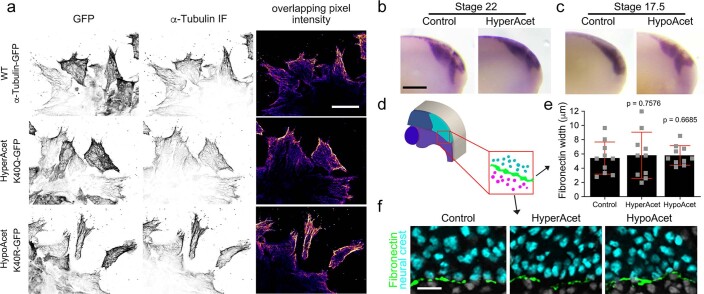
Extended Data Fig. 3. α-Tubulin constructs controls.
(a) Representative confocal projections showing the signal of wild type α-Tubulin-GFP and mutant α-Tubulin-GFP fused constructs with the signal of α-Tubulin, conditions as indicated. Note that the colocalization of α-Tubulin (IF, immunofluorescence) with the GFP signal of each construct (GFP) was extracted by using the ‘AND’ function of the ImageJ image calculator plugin. Then, colocalizing pixels were color-coded and presented in the right panel column as ‘overlapping pixel signal’ (scale bar 20 μm). (b, c) In situ hybridisation analysis of NC CCM in vivo, lateral views of sox8 hybridized embryos, treatments as indicated (scale bar 200 μm). Images are representative examples of three independent experiments (note that hyperacetylation blocks migration in vivo at stage 22 and hypoacetylation promotes premature migration already at stage 17.5). (d–e) Analysis of the impact of NC microtubule acetylation treatments on fibronectin thickness. (d) Diagram showing the regions measured to analyze NC grafts (cyan) impact on fibronectin (green) thickness at the interface with the head mesoderm (magenta). (e) Chart showing that the layer of Fibronectin has similar thickness in every treatment; bars represent the mean, and whiskers SD (two-tailed t-test, CI = 95%, n = 9 embryos); (f) representative confocal projections showing Fibronectin (green) between NC (cyan) and mesoderm (grey) (scale bar, 30 μm). a,b,c,f, representative images from at least 3 experiments.