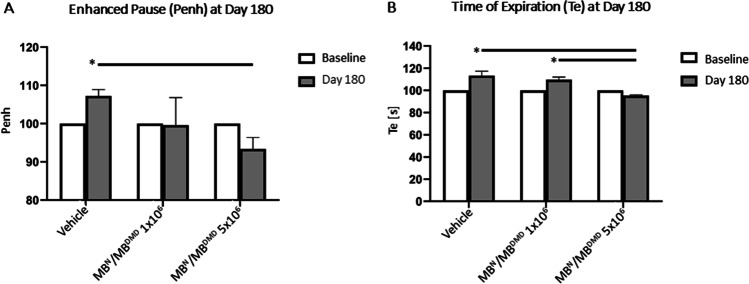
Fig. 7.
Systemic-intraosseous administration of human DEC results in reduced mdx-mediated respiratory disease at 180 days after transplant. Assessment of respiratory function by whole body plethysmography revealed: (A) significant decrease in enhanced pause (PENH) in 5 × 106 DEC-injected mice when compared to the vehicle-injected control at 180 days after DEC transplant (B) Significant decrease in expiration time (Te) was observed in both DEC-injected groups compared to the vehicle-injected controls and was dose dependent, confirming protective effect of DEC therapy on pulmonary function. Data were normalized to individual animals' Penh and Te values at baseline (starting at 100% at a baseline for all of the groups, as indicated by the white bars). Data presented as mean ± SEM; Two sample t-test assuming unequal variances. Buxco Instrument. Abbreviations: p < 0.05