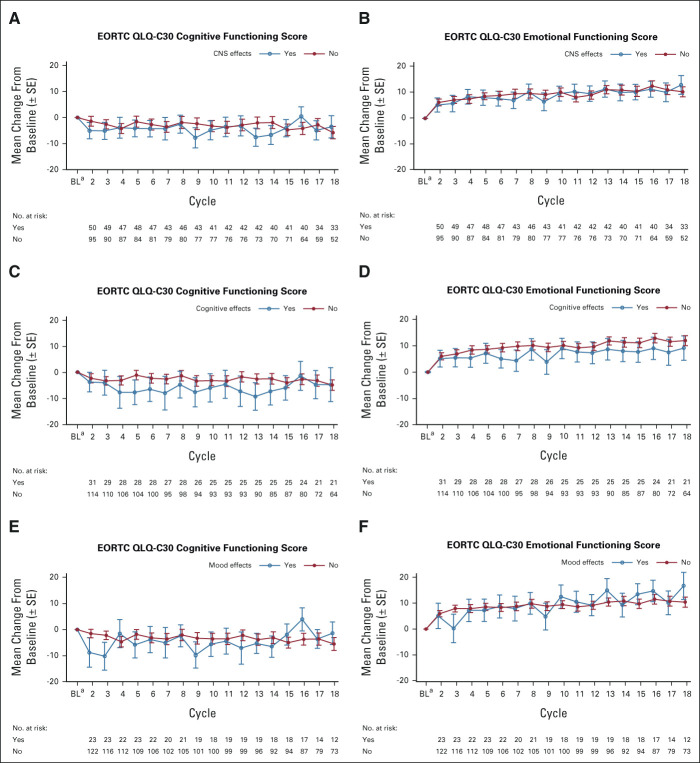
FIG A4.
Changes from baseline in EORTC QLQ-C30 Cognitive and Emotional Functioning scores with lorlatinib by (A and B) CNS adverse events; (C and D) cognitive effects; and (E and F) mood effects cluster terms (PRO population). The PRO population consisted of all randomly assigned patients who completed a baseline assessment and at least one postbaseline assessment. COGNITIVE EFFECTS were any events from HLGT Cognitive and attention disorders and disturbances, Deliria (including confusion) or Mental impairment disorders; MOOD EFFECTS were any events from HLGT Anxiety disorders and symptoms, Depressed mood disorders and disturbances, Manic and bipolar mood disorders and disturbances, Mood disorders and disturbances not elsewhere classified, or Personality disorders and disturbances in behavior. aBL was defined as the last assessment performed on or before the date of the first dose of study treatment. CNS effects defined as any event from the cognitive effects, mood effects, speech effects, or psychotic effects cluster terms. BL, baseline; EORTC QLQ-C30, European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; HLGT, high-level group terms; PRO, patient-reported outcome.