PURPOSE
Seropositivity for the HPV16-E6 oncoprotein is a promising marker for early detection of oropharyngeal cancer (OPC), but the absolute risk of OPC after a positive or negative test is unknown.
METHODS
We constructed an OPC risk prediction model that integrates (1) relative odds of OPC for HPV16-E6 serostatus and cigarette smoking from the human papillomavirus (HPV) Cancer Cohort Consortium (HPVC3), (2) US population risk factor data from the National Health Interview Survey, and (3) US sex-specific population rates of OPC and mortality.
RESULTS
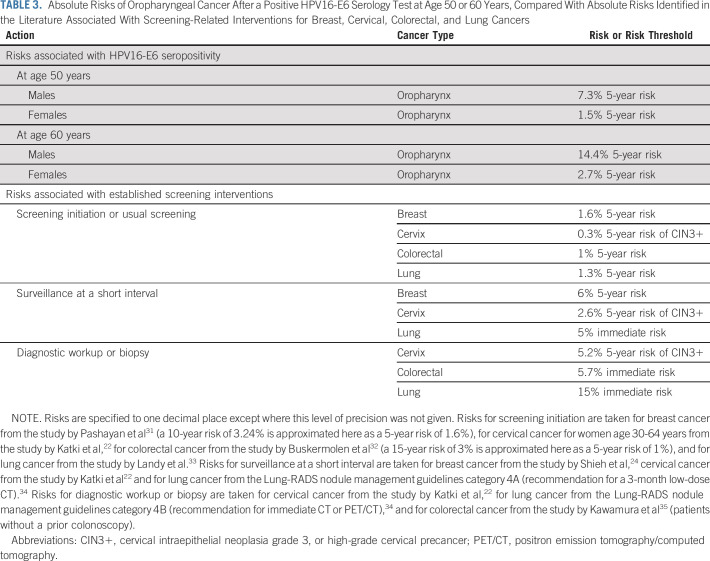
The nine HPVC3 cohorts included 365 participants with OPC with up to 10 years between blood draw and diagnosis and 5,794 controls. The estimated 10-year OPC risk for HPV16-E6 seropositive males at age 50 years was 17.4% (95% CI, 12.4 to 28.6) and at age 60 years was 27.1% (95% CI, 19.2 to 45.4). Corresponding 5-year risk estimates were 7.3% and 14.4%, respectively. For HPV16-E6 seropositive females, 10-year risk estimates were 3.6% (95% CI, 2.5 to 5.9) at age 50 years and 5.5% (95% CI, 3.8 to 9.2) at age 60 years and 5-year risk estimates were 1.5% and 2.7%, respectively. Over 30 years, after a seropositive result at age 50 years, an estimated 49.9% of males and 13.3% of females would develop OPC. By contrast, 10-year risks among HPV16-E6 seronegative people were very low, ranging from 0.01% to 0.25% depending on age, sex, and smoking status.
CONCLUSION
We estimate that a substantial proportion of HPV16-E6 seropositive individuals will develop OPC, with 10-year risks of 17%-27% for males and 4%-6% for females age 50-60 years in the United States. This high level of risk may warrant periodic, minimally invasive surveillance after a positive HPV16-E6 serology test, particularly for males in high-incidence regions. However, an appropriate clinical protocol for surveillance remains to be established.
INTRODUCTION
The incidence of oropharyngeal cancer (OPC) is increasing in the United States, despite decreasing rates of smoking- and alcohol-related OPC.1,2 This is driven by rapidly increasing incidence of OPC caused by human papillomavirus (HPV), marking a striking shift in the epidemiology of OPC in western countries.3,4 OPC incidence is five-fold higher in males than females,1 and projections indicate that the number of annual oropharyngeal cancers diagnosed in the United States will increase by nearly 50% between 2018 and 2045 despite HPV vaccination.5
CONTEXT
Key Objective
The incidence of human papillomavirus (HPV)–associated oropharyngeal cancer (OPC) is rising, and earlier detection could be beneficial. The HPV16-E6 blood-based biomarker has high sensitivity and specificity for HPV-driven OPC, but the absolute risk of OPC after a positive or negative HPV16-E6 test is unknown.
Knowledge Generated
We estimate that the 10-year risk of OPC for HPV16-E6 seropositive individuals is 17%-27% for males and 4%-6% for females at age 50-60 years. Corresponding risks for seronegative individuals are very low, ranging from 0.01% to 0.25% depending on age, sex, and smoking status.
Relevance (J.W. Friedberg)
-
The high risk of OPC among HPV16-E6 seropositive individuals warrants validation studies and development of minimally invasive surveillance protocols, particularly for males in regions where incidence rates reach levels that warrant such an intervention.*
*Relevance section written by JCO Editor-in-Chief Jonathan W. Friedberg, MD.
The majority of HPV-related OPCs are caused by HPV16.6 The survival over 4 years is estimated to be 92% for American Joint Committee on Cancer eighth edition stage I, 81% for stage II, and 62% for stage III,7 but there is substantial treatment-related morbidity.8-10 Given the increasing incidence of HPV-driven OPC, developing a strategy for early detection could be beneficial.11 A direct comparison of available assays and a recent review concluded that the most promising biomarker may be the HPV16-E6 oncoprotein.12,13 This marker is measured in plasma or serum and has estimated sensitivity over 90% and specificity over 99% for detecting HPV-driven OPC.14-19
The HPV Cancer Cohort Consortium (HPVC3) was established in 2015 with a central objective of better characterizing the HPV16-E6 biomarker. The first results from HPVC3 showed that seropositivity to HPV16-E6 can develop anytime between 6 and 28 years before OPC diagnosis, with a median lead time of 11.5 years.16 Once seropositivity develops, it is very uncommon to observe reversion to seronegativity, and continuous HPV16-E6 measurements provide little information beyond binary serostatus.16
Despite its remarkable performance characteristics, it is unclear whether HPV16-E6 could be implemented for early detection or screening for HPV-driven OPC.11,20 Because OPC is a relatively rare cancer, the risk of OPC after a positive HPV16-E6 serology test may not be sufficiently high to warrant additional follow-up or diagnostic tests. The results from the PLCO study suggested more than 6% 10-year risk for seropositive males and more than 3% for females,15 but these are likely underestimates because of the increase in OPC incidence since PLCO sample collection (1993-2001)1,2 and potential healthy volunteer effects.21
Absolute risk of cancer is used as a basis for action in multiple established screening strategies, including for cervical, breast, and lung cancers.22-24 Accurate and contemporary estimates of OPC risk among HPV16-E6 seropositive individuals are therefore needed to evaluate whether this biomarker could be implemented as part of a screening paradigm, and if so, what actions might be appropriate for individuals on the basis of their HPV16-E6 serostatus. We estimated the absolute risk of OPC after an HPV16-E6 serology test, on the basis of testing between age 40 and 70 years. We then compared these risk estimates with the levels of risk associated with specific actions in the context of screening for other cancers, such as short-interval surveillance or diagnostic workup.
METHODS
Overview
We constructed a risk prediction model anchored to contemporary population risks of OPC in the United States. The model accounts for competing mortality using non-OPC mortality rates. We generated odds ratios (ORs) for OPC according to risk factors including HPV16-E6 serostatus using data from HPVC3. Throughout the analysis, we defined OPC using the anatomic subsites listed in the Data Supplement and considered all OPCs regardless of HPV tumor status or histologic type.
The HPVC3
Participants with HPV-related cancers and control participants were selected from nine cohort studies in the United States, Europe, and Australia with blood samples collected between 1972 and 2009.16 Participants with specific HPV-related cancer types (oropharyngeal, anal, vaginal, and penile) were selected along with two to four controls per case, matched using incidence density sampling on cohort, study center, sex, date of blood collection, and date of birth. HPV16-E6 antibodies were measured in prediagnostic serum or plasma specimens at a single laboratory using multiplex serology, and the seropositivity threshold was 1,000 median fluorescence intensity.16,25,26 HPVC3 was approved by the IARC Ethics Committee and, as required, by institutional review boards for the participating cohorts.
For this study, we restricted to participants with OPC and all controls. Given our primary focus on a 10-year time horizon, we included participants with OPC if the time between blood draw and diagnosis was 10 years or less (n = 365). We included all controls (n = 5,794) because of the scarcity of HPV16-E6 seropositivity among cancer-free individuals.16 When repeat samples were available, we selected the sample drawn closest to diagnosis although selecting randomly from among the available samples had a negligible effect on the ORs (data not shown).
Statistical Analysis.
In overview, we constructed a prediction model to estimate OPC risk over 5, 10, and 30 years in categories specific to sex and the joint status of HPV16-E6 and smoking. We used the iCARE R package, which builds absolute risk models by synthesizing three sources of data.27 These inputs are described in detail below: (1) relative risks or odds for the association of risk factors with the outcome, (2) the distribution of risk factors in the population, and (3) age-specific population incidence rates for the outcome and for competing mortality. Using these inputs, the iCARE package constructs a model within a Cox proportional hazards framework with age as the underlying time metric of the baseline hazard function. In our analysis, the risk factor of interest was the joint status of HPV16-E6 and cigarette smoking (seronegative never-smokers, seronegative ever-smokers, and seropositive individuals). We estimated a single OR for HPV16-E6 seropositivity because this OR did not vary by smoking status (results below).
Relative odds of OPC.
We calculated two ORs for joint HPV16-E6 and smoking status using data from nine HPVC3 cohorts: one OR for HPV16-E6 seronegative ever-smokers and one OR for HPV16-E6 seropositive individuals, where the reference group was HPV16-E6 seronegative never-smokers. We estimated these ORs using an unconditional logistic regression model with the outcome of OPC and covariates of joint HPV16-E6/smoking status. Since all controls were included, we adjusted for matching factors including sex, cohort, and natural cubic splines for age. We used a single set of ORs for the analysis of males and females although the other analysis components were stratified by sex. Further details are provided in the Data Supplement Methods under Estimation of odds ratios.
Distribution of risk factors in the population.
We generated a data set describing the population distribution of risk factors using US nationally representative data from the National Health Interview Survey (NHIS) with accompanying statistical weights. Age, sex, and cigarette smoking status are collected in NHIS, and we stratified the data by sex. We estimated the population seroprevalence of HPV16-E6 by using a previously established methodology to reweight the 1,910 HPVC3 PLCO participants (ie, PLCO participants with an HPV16-E6 measurement, including participants with non-OPC cancers) to represent all PLCO intervention arm participants from whom they were sampled.15 Using this single seroprevalence estimate, we then assigned positive or negative HPV16-E6 serostatus to each individual in NHIS using a random binomial draw with uniform probability of success. Further details are provided in the Data Supplement Methods under Calculation of weights in the PLCO cohort and Data set describing the population distribution of risk factors.
Population incidence and mortality rates.
We downloaded sex- and age-specific incidence rates of OPC from the SEER program for 2010-2018, using the same definition of OPC described in the Data Supplement.28 We included 9 years of data to ensure stable rates. We downloaded sex- and age-specific population mortality rates, overall and for OPC, from the CDC Wide-ranging Online Data for Epidemiologic Research (WONDER) for 2010-2018.29 We estimated competing (non-OPC) mortality rates by subtracting OPC mortality from overall mortality.
We used a standard bootstrap procedure with 500 iterations to calculate 95% CIs for the risk estimates. The bootstrap allowed for variability, via sampling with replacement, in the ORs for HPV16-E6 and smoking and in the population seroprevalence for HPV16-E6. We treated the data from SEER, WONDER, and NHIS (other than HPV16-E6 serostatus) as fixed. Further details are provided in the Data Supplement Methods under Bootstrap procedure for confidence intervals.
Finally, we conducted two sensitivity analyses. The first restricted to the four HPVC3 cohorts from the United States (Table 1) before generating ORs for joint HPV16-E6 and smoking status. The second imputed the population HPV16-E6 seroprevalence estimated from a random sample in UK Biobank30 instead of using the estimate from the reweighted PLCO data.
TABLE 1.
Characteristics of Participants with OPC and Controls From the Human Papillomavirus Cancer Cohort Consortium Included in Analysis of Absolute OPC Risk
RESULTS
Our HPVC3 study sample included 365 participants with OPC and 5,794 controls (Table 1). Among participants with OPC, the median lead time between blood draw and diagnosis was 5.2 years (interquartile range, 2.4-7.7 years). Among all participants, 62% were male, the median age at blood draw was 57 years, and 66% had ever smoked cigarettes. The prediagnostic prevalence of HPV16-E6 seropositivity was 33% among participants with OPC and 0.3% among controls.
Compared with HPV16-E6 seronegative never-smokers, odds of OPC were increased among seronegative ever-smokers (OR = 3.2, 95% CI, 2.2 to 4.6) and HPV16-E6 seropositive individuals (OR = 403, 95% CI, 219 to 743). The OR for HPV16-E6 seropositivity was uniform by smoking status (OR = 395 and OR = 407 among never- and ever-smokers, P = .96). There was no evidence of heterogeneity in ORs across the cohorts for either HPV16-E6 seropositive individuals (Data Supplement, P = .25) or HPV16-E6 seronegative ever-smokers (Data Supplement, P = .51). The estimated population seroprevalence of HPV16-E6 on the basis of the weighted PLCO data was 0.66%. Using these parameters and the data inputs described in the Methods, the full model can be reconstructed using the iCARE package in R.27
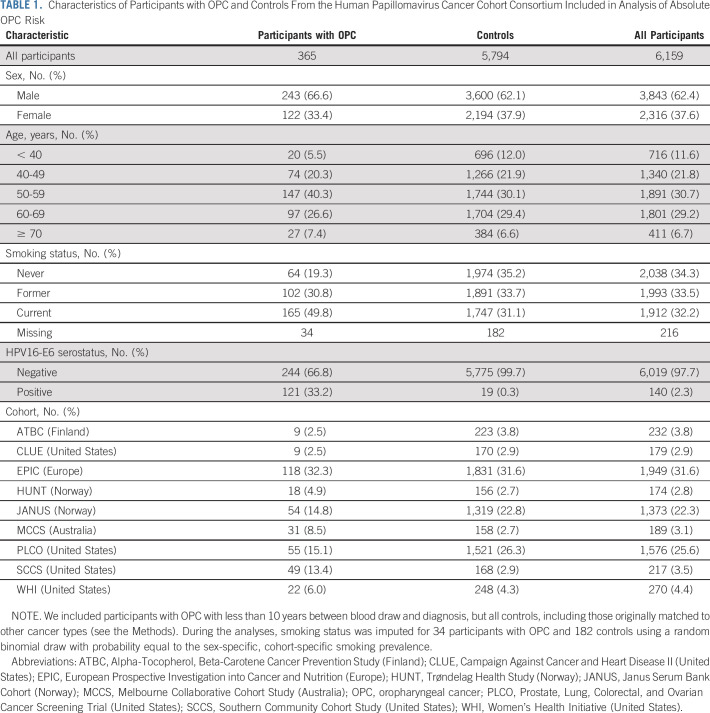
Estimated absolute risks of OPC for HPV16-E6 seropositive individuals were substantially higher for males than females. Risks are shown for an HPV16-E6 result at age 50 and 60 years in Figure 1 and for age 40 and 70 years in the Data Supplement. The 10-year risk estimate for HPV16-E6 seropositive males at age 50 years was 17.4% (95% CI, 12.4 to 28.6) and at age 60 years was 27.1% (95% CI, 19.2 to 45.4). For males at age 40 years, the 10-year risk was estimated to be 5.0% (95% CI, 3.6 to 8.4) and at age 70 years, it was 25.6% (95% CI, 17.7 to 47.1). For seropositive females, the 10-year risk estimate at age 50 years was 3.6% (95% CI, 2.5 to 5.9) and at age 60, it was 5.5% (95% CI, 3.8 to 9.2). The risk for females at age 40 years was estimated to be 1.2% (95% CI, 0.8 to 2.0), and at age 70 years, it was 6.0% (95% CI, 4.1 to 10.2). The reason for slightly higher risks among 60- versus 70-year-old males is the population incidence pattern of OPC, which peaks near age 65 years for males, but has a later and flatter peak among females (Data Supplement).
FIG 1.
Estimated absolute risk of OPC over 10 years after an HPV16-E6 serology result obtained at age 50 or 60 years. OPC, oropharyngeal cancer.
Considering a shorter time frame, 5-year risk estimates at age 40, 50, 60, and 70 years for HPV16-E6 seropositive males were 1.4% (95% CI, 1.0 to 2.4), 7.3% (95% CI, 5.1 to 12.3), 14.4% (95% CI, 10.0 to 25.4), and 15.6% (95% CI, 10.6 to 30.2), and for seropositive females were 0.4% (95% CI, 0.3 to 0.6), 1.5% (95% CI, 1.0 to 2.5), 2.7% (95% CI, 1.9 to 4.5), and 3.1% (95% CI, 2.2 to 5.4; Fig 1 and Data Supplement). We also considered a 30-year time frame to approximate lifetime risks. Over 30 years, the estimated risk among seropositive males tested at age 40 years was 39.9% (95% CI, 29.7 to 60.3) and at age 50 years, it was 49.9% (95% CI, 37.8 to 72.4), with corresponding risks for females estimated to be 9.6% (95% CI, 7.2 to 56.3) and 13.3% (95% CI, 10.0 to 68.1).
By contrast, estimated OPC risks for HPV16-E6 seronegative individuals were extremely low (Fig 1 and Data Supplement). For ever-smokers, 10-year risk estimates for males were 0.15% (95% CI, 0.09 to 0.23) for a negative test at age 50 years and 0.25% (95% CI, 0.16 to 0.36) at age 60 years. Corresponding risks for females were 0.03% (95% CI, 0.02 to 0.05) and 0.05% (95% CI, 0.03 to 0.07). For never-smokers, 10-year risk estimates for males were 0.05% (95% CI, 0.03 to 0.07) at age 50 years and 0.08% (95% CI, 0.04 to 0.12) at age 60 years. Corresponding risks for females were 0.009% (95% CI, 0.005 to 0.014) and 0.014% (95% CI, 0.007 to 0.022).
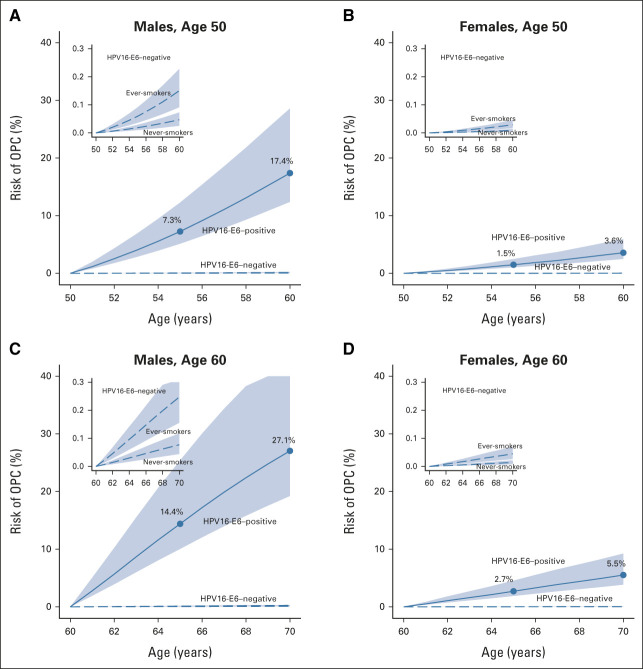
In a sensitivity analysis restricting to the four US cohorts to calculate ORs for joint HPV16-E6 and smoking status, estimated OPC risks were marginally lower, for example, 20.3% over 10 years for 60-year-old seropositive males compared with 27.1% in the primary analysis (Table 2). When we used the population HPV16-E6 seroprevalence estimated from a random sample in UK Biobank30 (0.98%, instead of 0.66% as estimated in PLCO), risks were similarly lower, eg, 21.9% over 10 years for 60-year-old seropositive males.
TABLE 2.
Results of Sensitivity Analyses Estimating the Absolute Risk for Oropharyngeal Cancer After an HPV16-E6 Test Result Obtained at Age 50 or 60 Years
DISCUSSION
The incidence of HPV-driven OPC is rising, and a comprehensive strategy for earlier detection could have an important public health impact. HPV16-E6 seropositivity is a highly sensitive and specific biomarker for future OPC. We estimated that the 10-year risk of OPC for HPV16-E6 seropositive individuals is 17%-27% for males and 4%-6% for females at age 50-60 years, with risk of OPC for seronegative individuals ranging from 0.01% to 0.25%. For a positive serology test at age 50 years, we estimated risk over 30 years to be 50% for males and 13% for females.
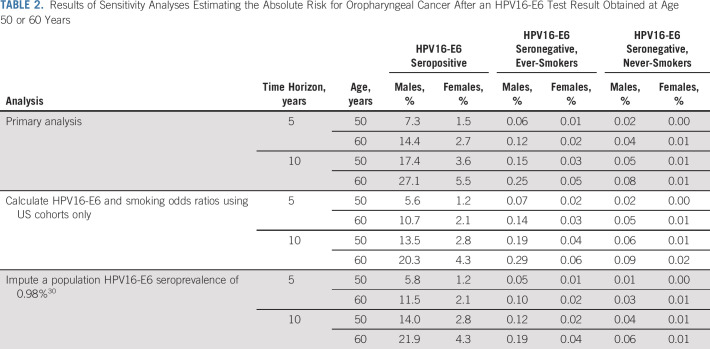
Table 3 reviews the absolute risks of cancer associated with specific actions in established screening strategies for breast, cervical, colorectal, and lung cancers. In general, screening is initiated or continued with the usual interval when individuals have a 5-year cancer risk of approximately 1%-2%.22,31-33 Surveillance at a short interval is associated with an approximately 6% 5-year risk of breast cancer, 3% 5-year risk of high-grade cervical precancer, or 5% immediate risk of lung cancer.22,24,34 These comparisons can contextualize the risks of OPC after HPV16-E6 seropositivity. We focus on age 50 years, because risks at age 40 years are low (Data Supplement) and approximately half of OPC cases have already been diagnosed at age 60 years.36 We found that the 5-year risk of OPC among 50-year-old HPV16-E6 seropositive males was 7.3%, which is comparable with the range of risks associated with short-interval surveillance for other cancers. For females, the 5-year 1.5% risk at age 50 years falls among accepted risks for usual screening or potentially short-interval surveillance.
TABLE 3.
Absolute Risks of Oropharyngeal Cancer After a Positive HPV16-E6 Serology Test at Age 50 or 60 Years, Compared With Absolute Risks Identified in the Literature Associated With Screening-Related Interventions for Breast, Cervical, Colorectal, and Lung Cancers
Taken together, this suggests that for males age 50 years and possibly for females, a hypothetical screening strategy might use HPV16-E6 serology as a rule-in test to define a population requiring periodic surveillance. An initial workup for people with a seropositive result could identify prevalent cancers, whereas periodic surveillance at a prespecified interval could identify incident cases. For males with a seronegative result, it might be reasonable to repeat the test every 5 or 10 years up to a certain age threshold since many people seroconvert to HPV16-E6 less than 10 years before OPC diagnosis and many OPCs occur at age 60-80 years.16
For periodic surveillance to identify incident cancers, it is unclear what test or combination of tests could be used and at what frequency. The strategy would need to be minimally invasive with few harms and high sensitivity, and potential options might include imaging or testing with a biomarker of short-term OPC risk. A recent study of 51 patients with known or suspected OPC found sensitivities of 90% for transcervical ultrasound, 69% for computed tomography, and 83% for positron emission tomography/computed tomography.37 Ultrasound is a promising technique,38 but its sensitivity for patients with unknown OPC status and ability to facilitate early detection are unclear.
For biomarkers, future research might aim to identify a blood-based or other noninvasive test that becomes positive approximately 1-3 years before OPC diagnosis. If such a biomarker could be identified, it could be measured at regular intervals among HPV16-E6 seropositive people to indicate when more invasive examination is warranted. Circulating tumor HPV DNA can have high sensitivity and specificity for detecting HPV-positive OPC at the time of diagnosis,39 and a recent study supports feasibility in a prediagnostic setting,40 but studies with larger numbers of prediagnostic samples from HPV-positive OPC cases are critically needed. A compelling analogy is Epstein-Barr virus DNA detection in plasma, which can facilitate early detection of nasopharyngeal cancer.41 Measurements of some proteins also differ between OPC cases and controls, including for HPV-positive OPCs.42-44 Other possible biomarkers could include circulating tumor DNA with mutations commonly found in HPV-related head and neck cancer,45 oral HPV DNA in saliva,46,47 or HPV-related antibodies to proteins other than E6.19 Although some of these approaches are promising, they are currently not sufficiently well-characterized, and further research will likely show that some are ill-suited for implementation. Ongoing studies such as SPANC and HOUSTON have begun to provide evidence about the feasibility of surveillance for HPV16-E6 seropositive individuals.47,48
We emphasize that our comparison with actionable risks for other cancer types is useful for contextualization, but there are important differences to consider. Survival for locally advanced HPV-related OPC is high compared with lung cancer, for example. The ability to identify early-stage disease or precursors is well-established for breast, cervical, colon, and lung cancers, but for OPC, this will require additional research and evaluation of potential diagnostic tools. Although OPC risk among HPV16-E6 seropositive people is high, from a public health perspective, the population risk of OPC remains lower than that for cancers where screening has historically been considered.49 Importantly, HPV16-E6 seropositivity also indicates increased risk for other HPV-related cancers, particularly anal cancer.26 An important next step is therefore to quantify the absolute risk of anal cancer among HPV16-E6 seropositive males and females, and ideally the risks of other HPV-related genital cancers as well, although their relationship with HPV16-E6 is weaker.26
Using our results, it is possible to speculate regarding hypothetical outcomes of population testing for HPV16-E6. Among 100,000 individuals, 660 (0.66%) might be HPV16-E6 seropositive. Among 50-year-old males, with a 30-year OPC risk of 48.1%, the program might identify 317 OPCs by age 80 years, with 315 males tested to identify one case. Among 50-year-old females, with a 30-year OPC risk of 16.6%, 110 cases might be identified, with 909 females tested to identify one case. For breast cancer screening, approximately 18 women are screened from age 50-70 years to identify one case, excluding overdiagnosis.50 This scenario differs from hypothetical HPV16-E6–based screening, in which approximately 99% of tested individuals would be seronegative and require little or no follow-up, whereas women in breast cancer screening undergo continuous screening. Still, these calculations illustrate the challenge to screen efficiently for a relatively rare disease like OPC and highlight that many considerations are needed.
The estimate of population HPV16-E6 seroprevalence has a strong influence on our risk estimates. Data to support or refute our estimate of 0.66% are scarce; however, one recent study tested 9,695 randomly selected UK Biobank participants and found a population seroprevalence of 0.98%, without differences by sex, age, or smoking status.30 When we used this estimate in a sensitivity analysis, risk estimates for HPV16-E6 seropositive individuals were modestly reduced (Table 2). In a smaller study of 553 US men age 50-64 years, 1.1% (n = 6) were seropositive.47 We emphasize that our assumption of constant HPV16-E6 seroprevalence across groups of older adults, while supported by the UK Biobank results,30 is difficult to validate and may not hold for the general US population.
One limitation of our statistical approach is that it assumes zero risk at the time of the HPV16-E6 serology test. In reality, some seropositive individuals would have prevalent disease, but data are lacking to quantify this risk. In the future, our approach could be updated using prevalence-incidence mixture models to account for prevalent disease.51 Our estimates may be roughly generalizable to many western countries, but in regions with lower rates of HPV-driven OPC, risk among HPV16-E6 seropositive people would be lower. New estimates should be generated as the epidemiology of OPC evolves.36 Finally, the lack of external validation of our model is a limitation of our study and a direction for future research. Since both HPV16-E6 seropositivity and OPC are rare observations, future validation would ideally involve testing a large cohort for HPV16-E6 seropositivity at baseline and assessing incident cases of OPC during a 10-year follow-up.
In conclusion, we estimate that the 10-year risk of OPC for HPV16-E6 seropositive individuals is approximately 17%-27% for males and 4%-6% for females at age 50-60 years. Corresponding risks for seronegative individuals are extremely low, ranging from 0.01%-0.25%. This suggests that a hypothetical screening strategy for OPC might use HPV16-E6 as a rule-in test to identify high-risk individuals who could benefit from periodic surveillance with a minimally invasive imaging or biomarker test. An important avenue for future work is to identify and characterize the utility of potential tests for surveillance among HPV16-E6 seropositive individuals.
ACKNOWLEDGMENT
We are grateful to all the participants in the HPVC3 cohorts. We thank Dr Parichoy Pal Choudhury for his assistance with the iCARE package. We thank Dr Douglas Lowy and Dr John Schiller for their feedback on the manuscript.
DISCLAIMER
Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/WHO.
SUPPORT
HPVC3 was funded by a grant from the US National Cancer Institute (grant: 5U01CA195603-02), with additional support from the intramural program of the Division of Cancer Epidemiology and Genetics, US NCI.
MCCS cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further augmented by Australian NHMRC grants (209057, 396414, and 1074383) and by infrastructure provided by Cancer Council Victoria. Cases and their vital status were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database. The Southern Community Cohort Study (SCCS) was supported by a grant from the US National Cancer Institute (grant: U01CA202979). Involvement of the Women's Health Initiative (WHI) collaborators was supported in part by National Cancer Institute P30 grants to the Roswell Park Comprehensive Cancer Institute (CA016056) and Einstein Cancer Research Center (CA013330). The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, and 75N92021D00005. The Trøndelag Health Study (HUNT) is a collaboration between the HUNT Research Center (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology NTNU), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health. We acknowledge the State of Maryland, the Maryland Cigarette Restitution Fund, and the National Program of Cancer Registries of the Centers for Disease Control and Prevention for the funds that support the collection and availability of the cancer registry data.
M.J. and P.B. contributed equally to this work as joint senior authors.
AUTHOR CONTRIBUTIONS
Conception and design: Hilary A. Robbins, Tim Waterboer, Aimée R. Kreimer, Mattias Johansson, Paul Brennan
Financial support: Tim Waterboer, Aimée R. Kreimer, Mattias Johansson, Paul Brennan
Collection and assembly of data: Aida Ferreiro-Iglesias, Tim Waterboer, Nicole Brenner, Mari Nygard, Noemi Bender, Lea Schroeder, Allan Hildesheim, Michael Pawlita, Gypsyamber D'Souza, Kala Visvanathan, Hilde Langseth, Nicolas F. Schlecht, Lesley F. Tinker, Ilir Agalliu, Sylvia Wassertheil-Smoller, Eivind Ness-Jensen, Kristian Hveem, Sara Grioni, Rudolf Kaaks, Maria-Jose Sánchez, Elisabete Weiderpass, Graham G. Giles, Roger L. Milne, Qiuyin Cai, William J. Blot, Wei Zheng, Stephanie J. Weinstein, Demetrius Albanes, Wen-Yi Huang, Neal D. Freedman
Data analysis and interpretation: Hilary A. Robbins, Mattias Johansson, Paul Brennan
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Absolute Risk of Oropharyngeal Cancer After an HPV16-E6 Serology Test and Potential Implications for Screening: Results From the Human Papillomavirus Cancer Cohort Consortium
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
No potential conflicts of interest were reported.
REFERENCES
- 1.Zumsteg ZS, Cook-Wiens G, Yoshida E, et al. : Incidence of oropharyngeal cancer among elderly patients in the United States. JAMA Oncol 2:1617-1623, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Xu L, Dahlstrom KR, Lairson DR, et al. : Projected oropharyngeal carcinoma incidence among middle-aged US men. Head Neck 41:3226-3234, 2019 [DOI] [PubMed] [Google Scholar]
- 3.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. : Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29:4294-4301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosetti C, Carioli G, Santucci C, et al. : Global trends in oral and pharyngeal cancer incidence and mortality. Int J Cancer 147:1040-1049, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Fakhry C, D'Souza G: Projected association of human papillomavirus vaccination with oropharynx cancer incidence in the US, 2020-2045. JAMA Oncol 7:e212907, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza G, Gross ND, Pai SI, et al. : Oral human papillomavirus (HPV) infection in HPV-positive patients with oropharyngeal cancer and their partners. J Clin Oncol 32:2408-2415, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhan KY, Eskander A, Kang SY, et al. : Appraisal of the AJCC 8th edition pathologic staging modifications for HPV-positive oropharyngeal cancer, a study of the National Cancer Data Base. Oral Oncol 73:152-159, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Fakhry C, Psyrri A, Chaturvedhi A: HPV and head and neck cancers: State-of-the-science. Oral Oncol 50:353-355, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Patel MA, Blackford AL, Rettig EM, et al. : Rising population of survivors of oral squamous cell cancer in the United States. Cancer 122:1380-1387, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Høxbroe Michaelsen S, Grønhøj C, Høxbroe Michaelsen J, et al. : Quality of life in survivors of oropharyngeal cancer: A systematic review and meta-analysis of 1366 patients. Eur J Cancer 78:91-102, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Kreimer AR, Shiels MS, Fakhry C, et al. : Screening for human papillomavirus-driven oropharyngeal cancer: Considerations for feasibility and strategies for research. Cancer 124:1859-1866, 2018 [DOI] [PubMed] [Google Scholar]
- 12.D’Souza G, Clemens G, Troy T, et al. : Evaluating the utility and prevalence of HPV biomarkers in oral rinses and serology for HPV-related oropharyngeal cancer. Cancer Prev Res 12:689-700, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balachandra S, Kusin SB, Lee R, et al. : Blood-based biomarkers of human papillomavirus–associated cancers: A systematic review and meta-analysis. Cancer 127:850-864, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kreimer AR, Johansson M, Waterboer T, et al. : Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 31:2708-2715, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreimer AR, Johansson M, Yanik EL, et al. : Kinetics of the human papillomavirus type 16 E6 antibody response prior to oropharyngeal cancer. J Natl Cancer Inst 109:djx005, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreimer AR, Ferreiro-Iglesias A, Nygard M, et al. : Timing of HPV16-E6 antibody seroconversion before OPSCC: Findings from the HPVC3 consortium. Ann Oncol 30:1335-1343, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang Kuhs KA, Pawlita M, Gibson SP, et al. : Characterization of human papillomavirus antibodies in individuals with head and neck cancer. Cancer Epidemiol 42:46-52, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lang Kuhs KA, Kreimer AR, Trivedi S, et al. : Human papillomavirus 16 E6 antibodies are sensitive for human papillomavirus-driven oropharyngeal cancer and are associated with recurrence. Cancer 123:4382-4390, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holzinger D, Wichmann G, Baboci L, et al. : Sensitivity and specificity of antibodies against HPV16 E6 and other early proteins for the detection of HPV16-driven oropharyngeal squamous cell carcinoma. Int J Cancer 140:2748-2757, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Day AT, Fakhry C, Tiro JA, et al. : Considerations in human papillomavirus-associated oropharyngeal cancer screening: A review. JAMA Otolaryngol Head Neck Surg 146:656-664, 2020 [DOI] [PubMed] [Google Scholar]
- 21.Pinsky PF, Miller A, Kramer BS, et al. : Evidence of a healthy volunteer effect in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Epidemiol 165:874-881, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Katki HA, Schiffman M, Castle PE, et al. : Benchmarking CIN3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis 17:S28-S35, 2013. (5 suppl 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Cancer Programme : Targeted Screening for Lung Cancer With Low Radiation Dose Computed Tomography. Standard Protocol Prepared for the NHS England Targeted Lung Health Checks Programme. Version 1, 2019. https://www.england.nhs.uk/wp-content/uploads/2019/02/targeted-Lu [Google Scholar]
- 24.Shieh Y, Eklund M, Madlensky L, et al. : Breast cancer screening in the precision medicine era: Risk-based screening in a population-based trial. J Natl Cancer Inst 109:djw290, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Waterboer T, Sehr P, Michael KM, et al. : Multiplex human papillomavirus serology based on in situ-purified glutathione S-transferase fusion proteins. Clin Chem 51:1845-1853, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Kreimer AR, Brennan P, Lang Kuhs KA, et al. : Human papillomavirus antibodies and future risk of anogenital cancer: A nested case-control study in the European Prospective Investigation Into Cancer and Nutrition Study. J Clin Oncol 33:877-884, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal Choudhury P, Maas P, Wilcox A, et al. : iCARE: An R package to build, validate and apply absolute risk models. PLoS One 15:e0228198, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Cancer Institute : Surveillance, Epidemiology, and End Results Program. www.seer.cancer.gov [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) : CDC WONDER Online Databases, 2020. https://wonder.cdc.gov [Google Scholar]
- 30.Brenner N, Mentzer AJ, Hill M, et al. : Characterization of human papillomavirus (HPV) 16 E6 seropositive individuals without HPV-associated malignancies after 10 years of follow-up in the UK Biobank. EBioMedicine 62:103123, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pashayan N, Morris S, Gilbert FJ, et al. : Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer. JAMA Oncol 4:1504-1510, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buskermolen M, Cenin DR, Helsingen LM, et al. : Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: A microsimulation modelling study. BMJ 367:l5383, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Landy R, Cheung LC, Berg CD, et al. : Contemporary implications of U.S. Preventive Services Task Force and risk-based guidelines for lung cancer screening eligibility in the United States. Ann Intern Med 171:384-386, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American College of Radiology : Lung CT Screening Reporting and Data System (Lung-RADS). https://www.acr.org/Quality-Safety/Resources/LungRADS [Google Scholar]
- 35.Kawamura T, Nakamura S, Sone D, et al. : Risk of colorectal cancer for fecal immunochemistry test-positive, average-risk patients after a colonoscopy. J Gastroenterol Hepatol 34:532-536, 2019 [DOI] [PubMed] [Google Scholar]
- 36.Cline BJ, Simpson MC, Gropler M, et al. : Change in age at diagnosis of oropharyngeal cancer in the United States, 1975-2016. Cancers (Basel) 12:3191, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang Kuhs KA, Wood CB, Wiggleton J, et al. : Transcervical sonography and human papillomavirus 16 E6 antibodies are sensitive for the detection of oropharyngeal cancer. Cancer 126:2658-2665, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coquia SF, Hamper UM, Holman ME, et al. : Visualization of the oropharynx with transcervical ultrasound. AJR Am J Roentgenol 205:1288-1294, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Damerla RR, Lee NY, You D, et al. : Detection of early human papillomavirus-associated cancers by liquid biopsy. JCO Precis Oncol 3:1-17, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rettig EM, Faden DL, Sandhu S, et al. : Detection of circulating tumor human papillomavirus DNA before diagnosis of HPV-positive head and neck cancer. Int J Cancer 151:1081-1085, 2022 [DOI] [PubMed] [Google Scholar]
- 41.Chan KCA, Woo JKS, King A, et al. : Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 377:513-522, 2017 [DOI] [PubMed] [Google Scholar]
- 42.Pereira LH, Reis IM, Reategui EP, et al. : Risk stratification system for oral cancer screening. Cancer Prev Res (Phila) 9:445-455, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramqvist T, Näsman A, Franzén B, et al. : Protein expression in tonsillar and base of tongue cancer and in relation to human papillomavirus (HPV) and clinical outcome. Int J Mol Sci 19:978, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JY, Shi T, Petyuk VA, et al. : Detection of head and neck cancer based on longitudinal changes in serum protein abundance. Cancer Epidemiol Biomarkers Prev 29:1665-1672, 2020 [DOI] [PubMed] [Google Scholar]
- 45.The Cancer Genome Atlas Network : Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517:576-582, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gipson BJ, Robbins HA, Fakhry C, et al. : Sensitivity and specificity of oral HPV detection for HPV-positive head and neck cancer. Oral Oncol 77:52-56, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dahlstrom KR, Anderson KS, Guo M, et al. : Screening for HPV-related oropharyngeal, anal, and penile cancers in middle-aged men: Initial report from the HOUSTON clinical trial. Oral Oncol 120:105397, 2021 [DOI] [PubMed] [Google Scholar]
- 48.Waterboer T, Brenner N, Gallagher R, et al. : Early detection of human papillomavirus–driven oropharyngeal cancer using serology from the Study of Prevention of Anal Cancer. JAMA Oncol 6:1806-1808, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Souza G, McNeel TS, Fakhry C: Understanding personal risk of oropharyngeal cancer: Risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol 28:3065-3069, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Independent UK Panel on Breast Cancer Screening : The benefits and harms of breast cancer screening: An independent review. Lancet 380:1778-1786, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Cheung LC, Pan Q, Hyun N, et al. : Mixture models for undiagnosed prevalent disease and interval-censored incident disease: Applications to a cohort assembled from electronic health records. Stat Med 36:3583-3595, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]