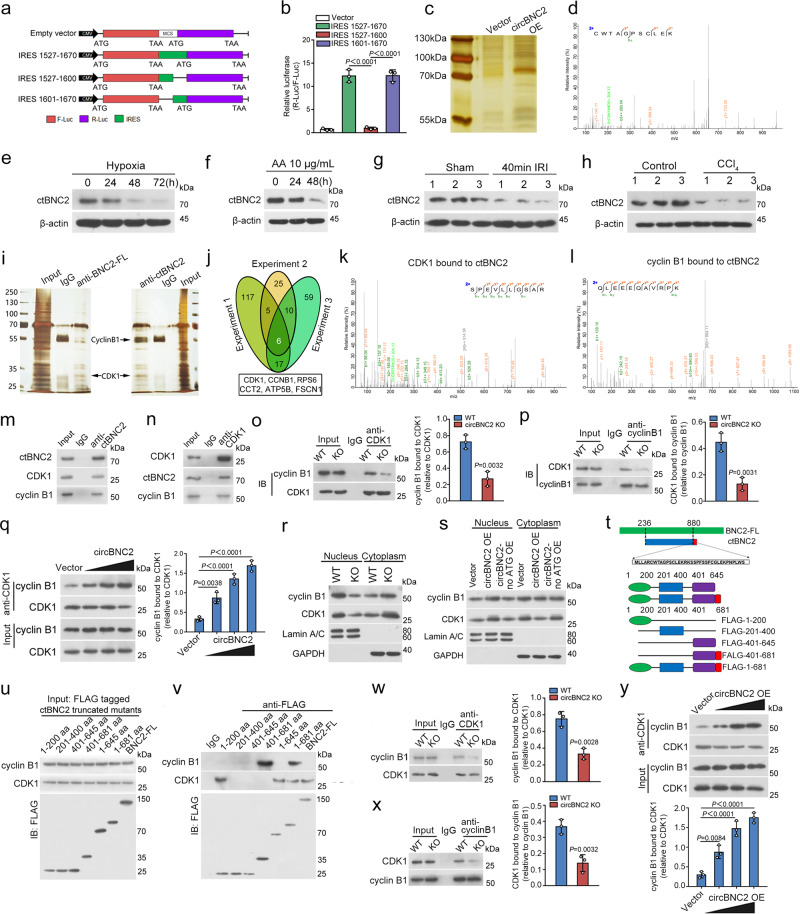
Fig. 4. circBNC2 promotes CDK1/cyclin B1 complex formation via encoding a 681-amino acid protein.
a, b IRES sequences in circBNC2 or its truncations were cloned between R-luc and F-Luc reporter genes (a) The predicted IRES activity in circBNC2 was tested by luciferase reporter assays (b). c Silver-staining of proteins from lysates of HK2 cells transfected with circBNC2 or empty vector. LC-MS analysis (samples from 3 independent experiments, n = 1 for each experiment) was used with the gel bands (70–100 kDa) from the lysates of HK2 cells overexpressing circBNC2. d Mass spectrometry result identifying a specific peptide sequence for circRNA translated BNC2 (ctBNC2). See also Supplementary Fig. 4a. e, f Western blots showing ctBNC2 expression in HK2 cells exposed to hypoxia (e) or AA (f) for the indicated timepoints. g, h Western blots showing ctBNC2 expression in cortex homogenates from mice with IRI-induced kidney fibrosis (g) and in liver homogenates from mice with CCl4-induced liver fibrosis (h). i The proteins in lysates of HK2 cells precipitated with anti-ctBNC2 or anti-full-length BNC2 (BNC2-FL) were detected by immunoprecipitation using normal IgG as the negative control. The SDS-PAGE gel with abundant bands (25–35 and 55–70 kDa) were collected and analyzed by Mass spectrometry to identify proteins interacted with ctBNC2 or BNC2-FL (3 independent experiments, n = 1 for each experiment). j A Venn diagram showing the intersection presenting 6 proteins (CDK1, cyclin B1, RPS6, ATP5B, CCT2 and Fascin) bound to ctBNC2 in three replicated experiments. k, l CDK1 and cyclin B1 bound to ctBNC2, as shown by Mass spectrometry results. See also Supplementary Data 1. m, n Interaction of ctBNC2 with CDK1 and cyclin B1, as shown by western blotting following immunoprecipitation of either ctBNC2 (m) or CDK1 (n). o, p Interaction of CDK1 with cyclin B1 was inhibited in circBNC2-KO HK2 cells, as shown by western blotting following immunoprecipitation of either CDK1 (o) or cyclin B1 (p). q Interaction of CDK1 with cyclin B1 was increased by overexpressing circBNC2 in 24-h hypoxia-treated HK2 cells in a dose-dependent manner, as shown by Immunoprecipitation assay followed by western blot. r Western blots showing CDK1 and cyclin B1 nuclear translocation was inhibited in circBNC2-KO HK2 cells. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. s Western blots showing CDK1 and cyclin B1 nuclear translocation in circBNC2 or circBNC2no ATG overexpressed HK2 cells treated by hypoxia for 24 h. t To test the binding site of ctBNC2 with CDK1 and cyclin B1, deletion mutants of ctBNC2 were established and tagged with FLAG. See also Supplementary Table 1. u, v Interaction of CDK1, cyclin B1 with ctBNC2 truncated mutants in vitro as shown by western blotting following immunoprecipitation of FLAG. w, x Interaction of CDK1 with cyclin B1 was inhibited in circBNC2-KO L-02 cells, as shown by western blotting following immunoprecipitation of either CDK1 (w) or cyclin B1 (x). y Interaction of CDK1 with cyclin B1 was increased by overexpressing circBNC2 in 24-h hypoxia-treated L-02 cells in a dose-dependent manner, as shown by western blots. For b, o, p, q, w–y, n = 3 biologically independent cells. Data are expressed as means ± SD. Two-sided T-test was used for the comparison of two groups (o, p, w, x). One-way ANOVA with Bonferroni post hoc test was used for comparison among multiple groups (b, q, y). Source data are provided as a Source Data file.