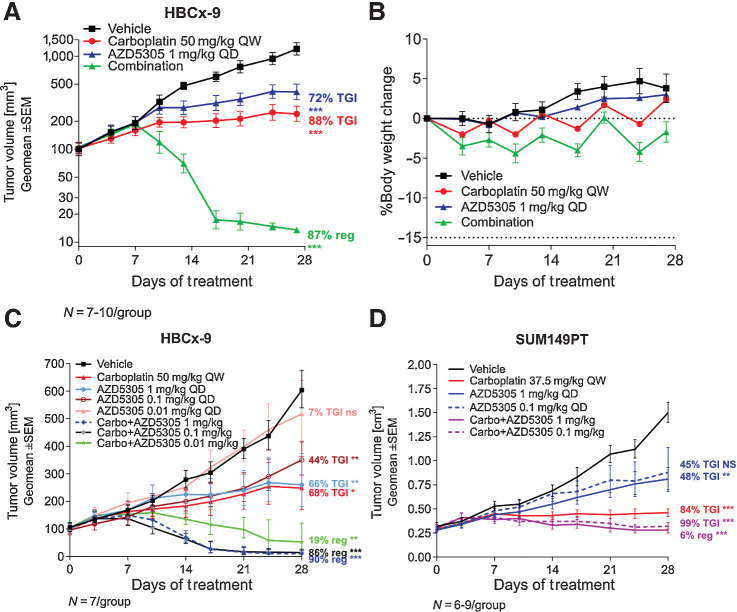
Figure 6.
Antitumor efficacy of AZD5305 in combination with carboplatin in vivo. A, Antitumor efficacy of AZD5305 in combination with carboplatin in the TNBC HBCx-9 PDX. B, Tolerability of the treatments was assessed by monitoring body weight changes throughout the treatment duration. C and D, Anti-tumor efficacy of dose response of AZD5305 in combination with carboplatin in HBCx-9 (C) and SUM149PT (D) tumor models. Mice were dosed with indicated dose levels of AZD5305 once daily orally (PO) and/or with carboplatin once weekly intraperitoneally (IP) for 4 weeks. Graphs depict geomean tumor volume ±SEM and percent tumor growth inhibition (TGI) or regression (reg). Statistical significance was evaluated compared to the vehicle group using a one-tailed t test (*, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001).