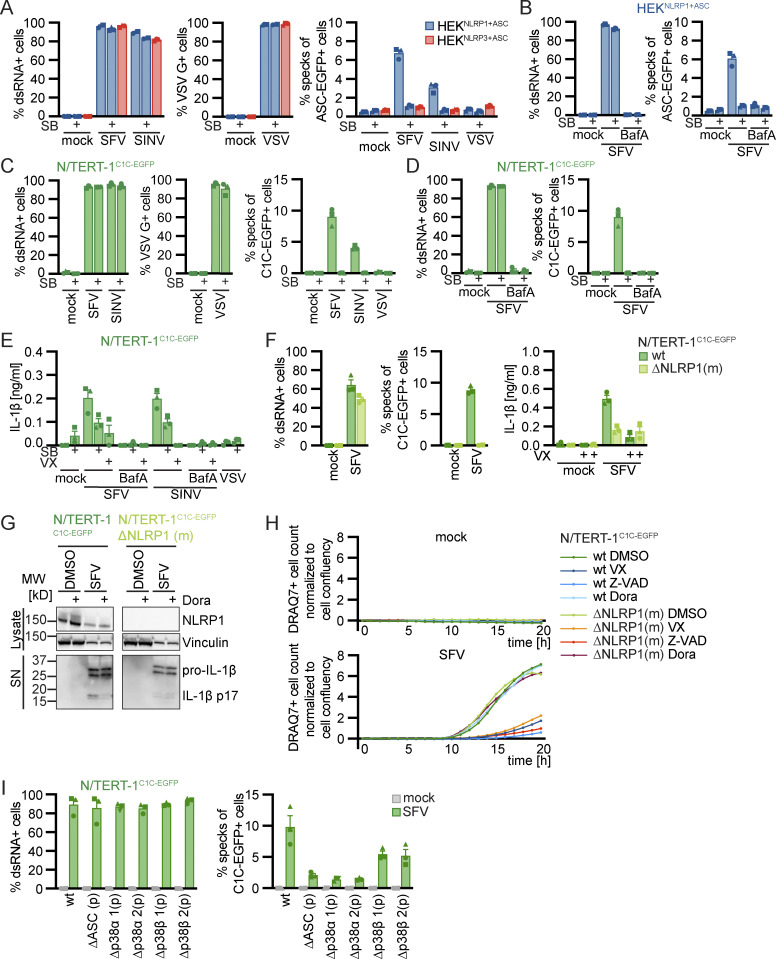
Figure 4.
Alphavirus infection activates human NLRP1 in a p38-dependent manner. (A–E) HEKNLRP1+ASC and HEKNLRP3+ASC (A and B) or N/TERT-1C1C-EGFP (C–E) cells were infected with SFV, SINV, or VSV at an MOI of 1 (HEK) or 5 (N/TERT-1) for 20 h in the presence or absence of 20 µM SB, where indicated in the presence of 100 nM BafA. Infected cells were stained with antibodies for dsRNA (SFV, SINV) or VSV G (VSV), and cells analyzed by flow cytometry. Speck assembly was quantified by flow cytometry as described in Fig. 1 (A–D). Specks were only quantified in infected cells for all samples treated with virus, i.e., not in mock controls. IL-1β from the supernatants of cells stimulated in the absence or presence of 100 μM VX was quantified by HTRF (E). (F–I) N/TERT-1C1C-EGFP cells (F–I), their monoclonal NLRP1 knockout derivative (F–H), or their polyclonal p38α or p38β knockout derivatives (I) were infected with SFV as in C–E. Infection, speck assembly (F, I) and IL-1β release (F) were quantified as in C–E. NLRP1 and Vinculin in the lysates, as well as IL-1β in precipitated supernatants were analyzed by immunoblot (G). The immunblot data from the complete experiment with additional samples and antibodies is shown in Fig. S4. Uptake of non-cell permeable DNA dye DRAQ7 was detected every hour for a total of 20 h, in the presence of DMSO, 100 μM VX, 50 μM Z-VAD, or 10 µM Dora (H). N/TERT-1C1C-EGFP cells were stimulated in the presence of 100 µM VX for all flow cytometry experiments. Data from all experiments quantifying specks or IL-1β release represents average values (with individual data points) from three independent experiments ± SEM. Immunoblots (G) and quantifications of DRAQ7 uptake over time (H) display data representative of two (G) or three (H) independent experiments. MW, molecular weight. Source data are available for this figure: SourceData F4.