Fig. 2.
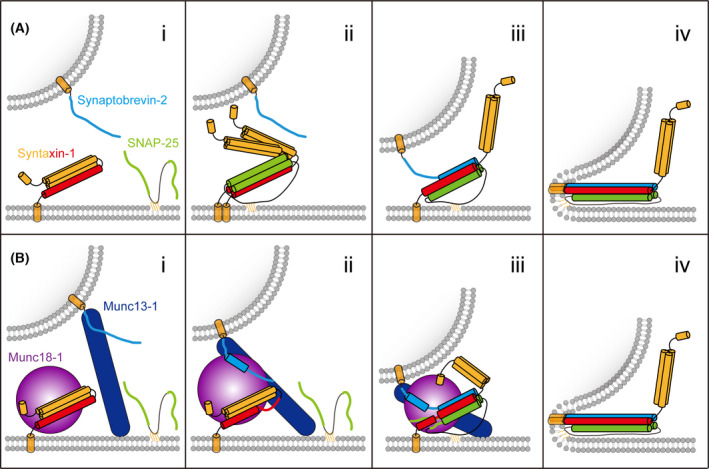
Models of SNARE‐mediated membrane fusion and synaptic exocytosis. (A) The zippering model with merely the three neuronal SNAREs. (i) At rest state, syntaxin‐1 adopts self‐inhibitory conformation; (ii) syntaxin‐1 fluctuates between closed and open conformations and is prone to form a 2 : 1 complex with SNAP‐25; (iii) synaptobrevin‐2 displaces one copy of syntaxin‐1 of the 2 : 1 complex; the N‐termini of the SNARE motifs nucleate together to promote complex assembly; (iv) zippering of the SNARE motifs transfers sufficient energy into the membrane thus catalyzing membrane fusion. (B) Munc18‐1 and Munc13‐1 synergistically organize neuronal SNARE complex assembly and synaptic exocytosis. (i) Munc18‐1 captures syntaxin‐1 into closed conformation; Munc13‐1 bridges the presynaptic membrane and synaptic vesicle to facilitate synaptic vesicle docking; (ii) Munc13‐1 interacts with Munc18‐1−syntaxin‐1 complex to induce conformational changes of the syntaxin‐1 linker region and Munc18‐1 domain 3a, leading to uncaging of the N‐terminus of the syntaxin‐1 SNARE motif and extension of Munc18‐1 domain 3a; in the meantime, Munc18‐1 interacts with the C‐terminal half of synaptobrevin‐2 with the assistance of the binding between Munc13‐1 and synaptobrevin‐2 JLR. This intermediate underlies a potential conformational state, namely the prefusion priming complex; (iii) the N‐termini of the SNARE motifs start to nucleate to produce a half‐zippered SNARE complex, which is organized by Munc18‐1 and Munc13‐1; (iv) full zippering of the SNARE motifs in response to calcium signal is accompanied by the interplay of complexins/synaptotagmin‐1 with the half‐zippered SNARE complex (not shown). The color schemes of the neuronal SNAREs are the same as in Fig. 1. Munc18‐1 and Munc13‐1 are colored in purple and navy blue, respectively.
