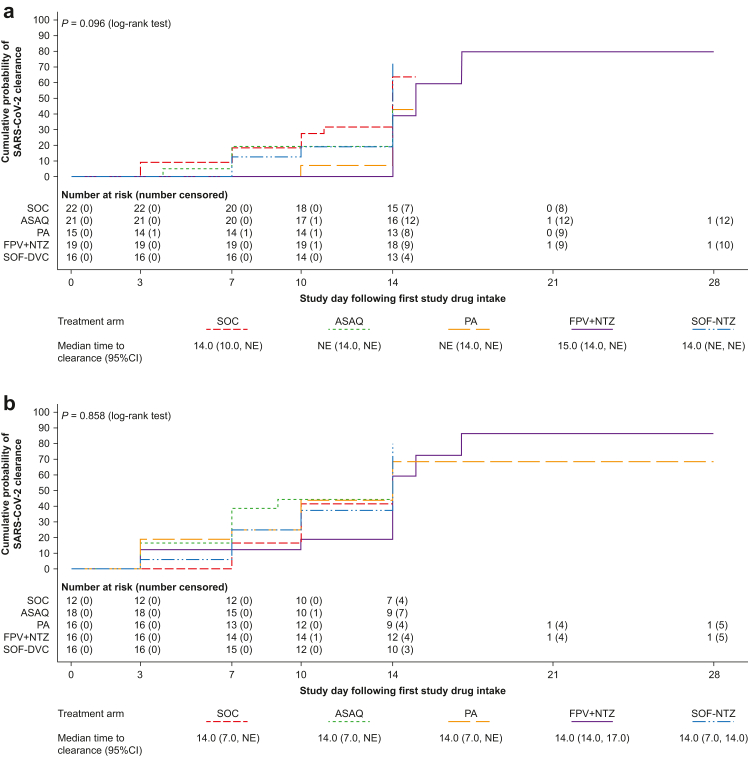
Fig. 3.
Time to SARS-CoV-2 clearance based on quantitative RT-PCR in patients with (a) high viral load (mITT population) and (b) high-risk patients. Shown are Kaplan–Meier curves. Time to clearance was defined as the time to the first negative quantitative SARS-CoV-2 RT-PCR test (collected post-baseline on days 3, 7, 10, 14, 21, and 28), without any subsequent positive quantitative SARS-CoV-2 RT-PCR test. Patients who withdrew from the study were censored on the day of withdrawal; patients with missing data were censored on the day of the last available data; patients without any post-baseline data were censored on day 1. mITT, modified intention-to-treat; NE, non-evaluable; ASAQ, artesunate-amodiaquine; PA, pyronaridine-artesunate; FPV + NTZ, favipiravir plus nitazoxanide; SOF-DCV, sofosbuvir-daclatasvir. High viral load was ≥175,145 copies/mL. High risk was defined as age >60 years or body mass index >30 kg/m2 plus the presence of at least one comorbidity for progression to severe disease.