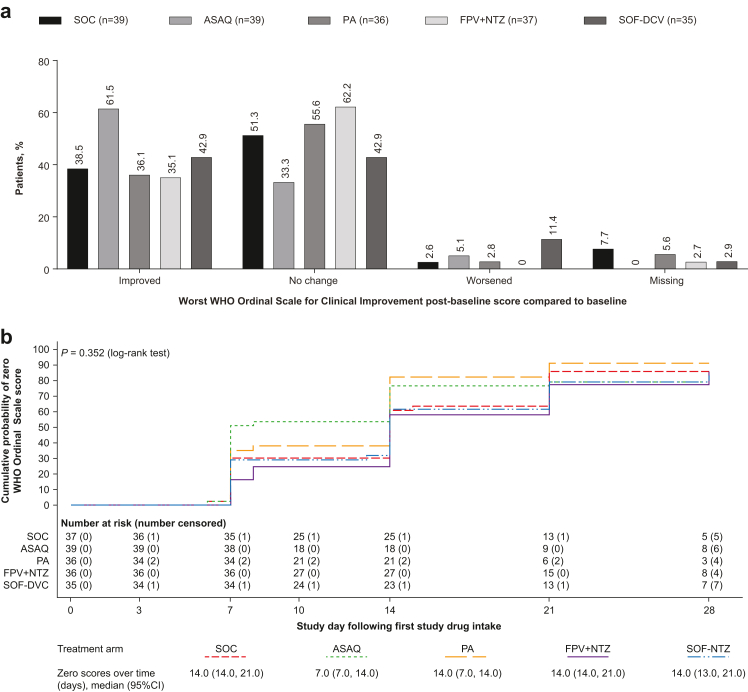
Fig. 5.
(a) Change from baseline in WHO Ordinal Scale for Clinical Improvement (b) Time to first zero score on the WHO Ordinal Scale for Clinical Improvement (mITT population). (a) Change from baseline was taken as the worst score at any time during the study. (b) Zero scores over time presents the time point (in days) by which the estimated cumulative probability of a zero WHO Ordinal Scale score was 50% (median) and the corresponding two-sided 95% confidence interval. Patients who withdrew from the study were censored on the day of withdrawal; patients with missing data were censored on the day of the last available data; patients without any post-baseline data were censored on day 1. mITT, modified intention-to-treat; ASAQ, artesunate-amodiaquine; PA, pyronaridine-artesunate; FPV + NTZ, favipiravir plus nitazoxanide; SOF-DCV, sofosbuvir-daclatasvir.