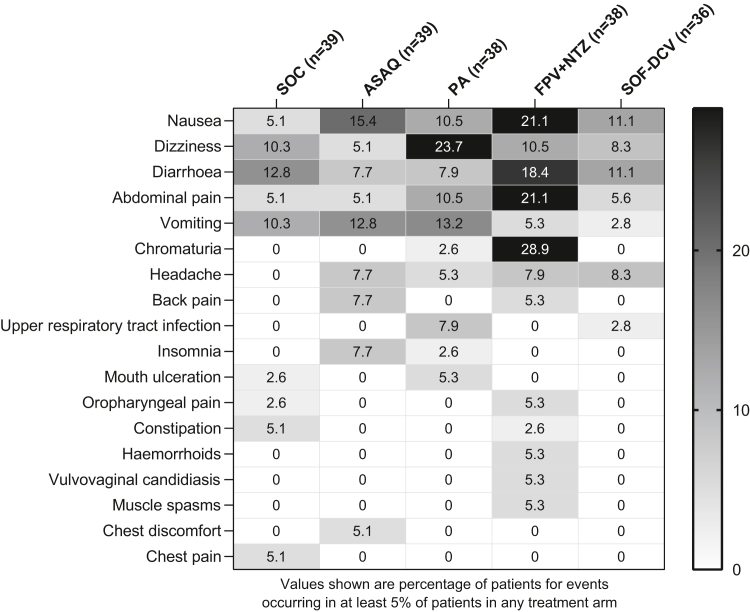
Fig. 6.
Frequency of the most common adverse events of any cause (safety population). Patients could have more than one adverse event. Adverse events were classified using MedDRA version 23.0. ASAQ, artesunate-amodiaquine; PA, pyronaridine-artesunate; FPV + NTZ, favipiravir plus nitazoxanide; SOF-DCV, sofosbuvir-daclatasvir.