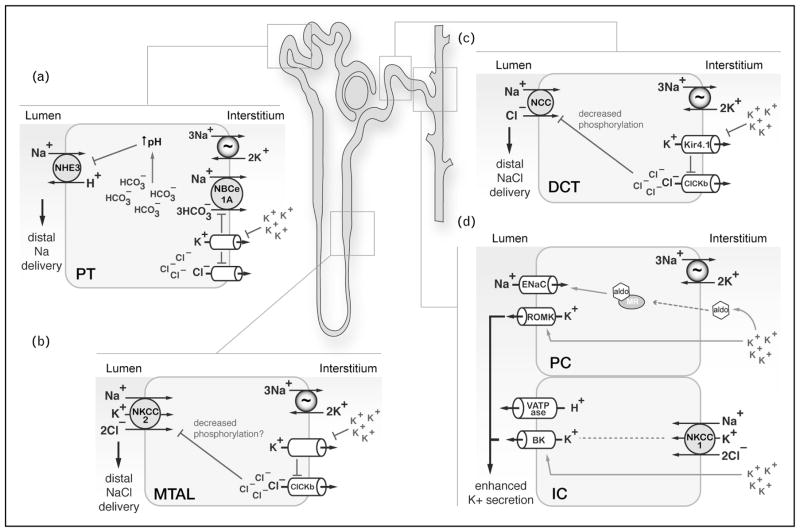
FIGURE 1.
The renal response to hyperkalemia involves multiple nephron segments. Summary of proposed transport events occurring in various sections of the nephron during dietary potassium loading. Current evidence suggests that in the proximal tubule, medullary thick ascending limb, and distal convoluted tubule, hyperkalemia-induced depolarization events at the basolateral membrane diminish transcellular sodium reabsorption, enhancing distal Na+ delivery. (a) A mathematical model [27▪] suggests that depolarization suppresses bicarbonate reabsorption via electrogenic sodium bicarbonate cotransporter 1A (NBCe1A), resulting in cytosolic alkalinization and reduced sodium–hydrogen exchanger activity. (b) In the medullary thick ascending limb, increased interstitial [K+] caused by enhanced medullary recycling diminishes sodium reabsorption, likely via NKCC2. It has been proposed that this process is also linked to basolateral membrane depolarization, which may downregulate NKCC2 activity via dephosphorylation, though this remains to be tested empirically. (c) In the distal convoluted tubule, hyperkalemia depolarization downregulates Kir4.1 activity, which increases intracellular chloride levels, attenuating sodium chloride cotransporter phosphorylation and activity. (d) Blockade of sodium transport in these regions of the nephron enhance NaCl delivery to principal cells and intercalated cells of the connecting tubule and cortical collecting duct, where voltage dependent K+ secretion via epithelial sodium channel and renal outer medullary K+ channel and flow dependent K+ secretion via BK channels occur, respectively. The enhanced K+ secretion in the connecting tubule and cortical collecting duct occurs through both aldosterone-dependent and aldosterone-independent processes.