Table 1.
Optimization of the Csp3-F bond transformation with lithium iodidea
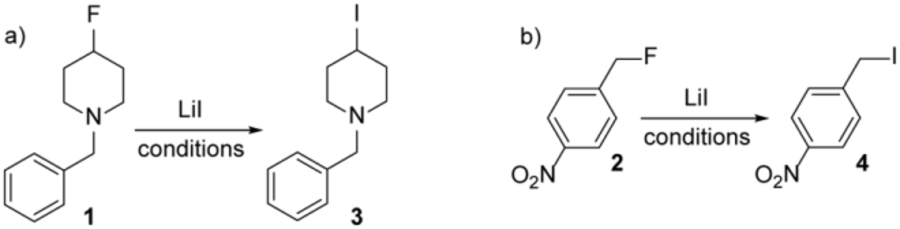
| |||||
|---|---|---|---|---|---|
| Entry | Substrate | Solvent | Time (h) | Product | Yield (%) |
| 1 | 1 | CH2Cl2 | 6 | 3 | 99 |
| 2 | 1 | CH2Cl2 | 6 | 3 | 80b |
| 3 | 1 | CHCl3 | 24 | 3 | 99 |
| 4 | 1 | ClCH2CH2Cl | 24 | 3 | 99 |
| 5 | 1 | DMF | 48 | 3 | 0 |
| 6 | 1 | DMSO | 48 | 3 | 0 |
| 7 | 1 | CH3CN | 48 | 3 | 0 |
| 8 | 1 | NMP | 48 | 3 | 0 |
| 9 | 1 | 1,4-dioxane | 48 | 3 | 0 |
| 10 | 1 | Et2O | 48 | 3 | 0 |
| 11 | 1 | MeOH | 48 | 3 | 0 |
| 12 | 1 | EtOH | 48 | 3 | 0 |
| 13 | 1 | pyridine | 48 | 3 | 0 |
| 14 | 1 | benzene | 48 | 3 | 98 |
| 15 | 1 | toluene | 48 | 3 | 65 |
| 16 | 1 | toluene | 6 | 3 | 99c |
| 17 | 1 | neat | 18 | 3 | 99d |
| 18 | 2 | CH2Cl2 | 48 | 4 | 99 |
| 19 | 2 | CH2Cl2 | 5 | 4 | 99e |
| 20 | 2 | toluene | 21 | 4 | 99 |
| 21 | 2 | toluene | 24 | 4 | 94e |
| 22 | 2 | toluene | 6 | 4 | 93c |
Conditions: 1 (0.2 mmol), LiI (0.4 mmol) in 0.4 mL solvent at 25 °C.
1.2 equiv. of LiI used,
The reaction was carried out at 60 °C,
The reaction was carried out in the absence of solvent with 3.0 equivalent of LiI,
1.0 equivalent of TEMPO was added.
